The coordination of cell growth during fission yeast mating requires Ras1-GTP hydrolysis
- PMID: 24147005
- PMCID: PMC3797800
- DOI: 10.1371/journal.pone.0077487
The coordination of cell growth during fission yeast mating requires Ras1-GTP hydrolysis
Expression of concern in
-
Expression of Concern: The Coordination of Cell Growth during Fission Yeast Mating Requires Ras1-GTP Hydrolysis.PLoS One. 2019 Oct 10;14(10):e0223817. doi: 10.1371/journal.pone.0223817. eCollection 2019. PLoS One. 2019. PMID: 31600315 Free PMC article. No abstract available.
Abstract
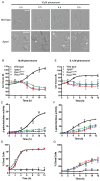
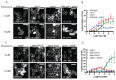
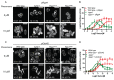
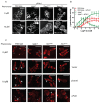
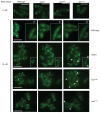
The spatial and temporal control of polarity is fundamental to the survival of all organisms. Cells define their polarity using highly conserved mechanisms that frequently rely upon the action of small GTPases, such as Ras and Cdc42. Schizosaccharomyces pombe is an ideal system with which to study the control of cell polarity since it grows from defined tips using Cdc42-mediated actin remodeling. Here we have investigated the importance of Ras1-GTPase activity for the coordination of polarized cell growth during fission yeast mating. Following pheromone stimulation, Ras1 regulates both a MAPK cascade and the activity of Cdc42 to enable uni-directional cell growth towards a potential mating partner. Like all GTPases, when bound to GTP, Ras1 adopts an active conformation returning to an inactive state upon GTP-hydrolysis, a process accelerated through interaction with negative regulators such as GAPs. Here we show that, at low levels of pheromone stimulation, loss of negative regulation of Ras1 increases signal transduction via the MAPK cascade. However, at the higher concentrations observed during mating, hyperactive Ras1 mutations promote cell death. We demonstrate that these cells die due to their failure to coordinate active Cdc42 into a single growth zone resulting in disorganized actin deposition and unsustainable elongation from multiple tips. These results provide a striking demonstration that the deactivation stage of Ras signaling is fundamentally important in modulating cell polarity.
Conflict of interest statement
Figures
Similar articles
-
Differential GAP requirement for Cdc42-GTP polarization during proliferation and sexual reproduction.J Cell Biol. 2018 Dec 3;217(12):4215-4229. doi: 10.1083/jcb.201806016. Epub 2018 Oct 2. J Cell Biol. 2018. PMID: 30279276 Free PMC article.
-
Exploration and stabilization of Ras1 mating zone: A mechanism with positive and negative feedbacks.PLoS Comput Biol. 2018 Jul 20;14(7):e1006317. doi: 10.1371/journal.pcbi.1006317. eCollection 2018 Jul. PLoS Comput Biol. 2018. PMID: 30028833 Free PMC article.
-
Transcriptional regulation of a Ras nucleotide-exchange factor gene by extracellular signals in fission yeast.J Cell Sci. 1994 Dec;107 ( Pt 12):3635-42. doi: 10.1242/jcs.107.12.3635. J Cell Sci. 1994. PMID: 7706412
-
Control of signal transduction and morphogenesis by Ras.Semin Cell Biol. 1995 Apr;6(2):89-94. doi: 10.1016/1043-4682(95)90005-5. Semin Cell Biol. 1995. PMID: 7548847 Review.
-
Spatial segregation of Ras signaling: new evidence from fission yeast.Cell Cycle. 2006 Sep;5(17):1936-9. doi: 10.4161/cc.5.17.3187. Epub 2006 Sep 1. Cell Cycle. 2006. PMID: 16931912 Free PMC article. Review.
Cited by
-
Inhibition of Ras activity coordinates cell fusion with cell-cell contact during yeast mating.J Cell Biol. 2018 Apr 2;217(4):1467-1483. doi: 10.1083/jcb.201708195. Epub 2018 Feb 16. J Cell Biol. 2018. PMID: 29453312 Free PMC article.
-
Differential GAP requirement for Cdc42-GTP polarization during proliferation and sexual reproduction.J Cell Biol. 2018 Dec 3;217(12):4215-4229. doi: 10.1083/jcb.201806016. Epub 2018 Oct 2. J Cell Biol. 2018. PMID: 30279276 Free PMC article.
-
Local Pheromone Release from Dynamic Polarity Sites Underlies Cell-Cell Pairing during Yeast Mating.Curr Biol. 2016 Apr 25;26(8):1117-25. doi: 10.1016/j.cub.2016.02.064. Epub 2016 Mar 24. Curr Biol. 2016. PMID: 27020743 Free PMC article.
-
Parameter identification problems in the modelling of cell motility.J Math Biol. 2015 Aug;71(2):399-436. doi: 10.1007/s00285-014-0823-6. Epub 2014 Sep 2. J Math Biol. 2015. PMID: 25174444
-
Optogenetics reveals Cdc42 local activation by scaffold-mediated positive feedback and Ras GTPase.PLoS Biol. 2020 Jan 24;18(1):e3000600. doi: 10.1371/journal.pbio.3000600. eCollection 2020 Jan. PLoS Biol. 2020. PMID: 31978045 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

