Biosynthesis of levan, a bacterial extracellular polysaccharide, in the yeast Saccharomyces cerevisiae
- PMID: 24147008
- PMCID: PMC3795680
- DOI: 10.1371/journal.pone.0077499
Biosynthesis of levan, a bacterial extracellular polysaccharide, in the yeast Saccharomyces cerevisiae
Abstract
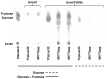
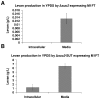
Levans are fructose polymers synthesized by a broad range of micro-organisms and a limited number of plant species as non-structural storage carbohydrates. In microbes, these polymers contribute to the formation of the extracellular polysaccharide (EPS) matrix and play a role in microbial biofilm formation. Levans belong to a larger group of commercially important polymers, referred to as fructans, which are used as a source of prebiotic fibre. For levan, specifically, this market remains untapped, since no viable production strategy has been established. Synthesis of levan is catalysed by a group of enzymes, referred to as levansucrases, using sucrose as substrate. Heterologous expression of levansucrases has been notoriously difficult to achieve in Saccharomyces cerevisiae. As a strategy, this study used an invertase (Δsuc2) null mutant and two separate, engineered, sucrose accumulating yeast strains as hosts for the expression of the levansucrase M1FT, previously cloned from Leuconostoc mesenteroides. Intracellular sucrose accumulation was achieved either by expression of a sucrose synthase (Susy) from potato or the spinach sucrose transporter (SUT). The data indicate that in both Δsuc2 and the sucrose accumulating strains, the M1FT was able to catalyse fructose polymerisation. In the absence of the predicted M1FT secretion signal, intracellular levan accumulation was significantly enhanced for both sucrose accumulation strains, when grown on minimal media. Interestingly, co-expression of M1FT and SUT resulted in hyper-production and extracellular build-up of levan when grown in rich medium containing sucrose. This study presents the first report of levan production in S. cerevisiae and opens potential avenues for the production of levan using this well established industrial microbe. Furthermore, the work provides interesting perspectives when considering the heterologous expression of sugar polymerizing enzymes in yeast.
Conflict of interest statement
Figures




Similar articles
-
Efficient production of levan using a recombinant yeast Saccharomyces cerevisiae hypersecreting a bacterial levansucrase.J Ind Microbiol Biotechnol. 2019 Nov;46(11):1611-1620. doi: 10.1007/s10295-019-02206-1. Epub 2019 Jun 22. J Ind Microbiol Biotechnol. 2019. PMID: 31230216
-
Catalytic biosynthesis of levan and short-chain fructooligosaccharides from sucrose-containing feedstocks by employing the levansucrase from Leuconostoc mesenteroides MTCC10508.Int J Biol Macromol. 2019 Apr 15;127:486-495. doi: 10.1016/j.ijbiomac.2019.01.070. Epub 2019 Jan 17. Int J Biol Macromol. 2019. PMID: 30659880
-
Cold induced expression of a novel levansucrase gene sacB1 enhances exopolysaccharide production and stress resilience in Leuconostoc mesenteroides.Sci Rep. 2025 Jul 2;15(1):22980. doi: 10.1038/s41598-025-04141-x. Sci Rep. 2025. PMID: 40595955 Free PMC article.
-
[Application of levansucrase in levan synthesis--a review].Wei Sheng Wu Xue Bao. 2014 Jun 4;54(6):601-7. Wei Sheng Wu Xue Bao. 2014. PMID: 25272807 Review. Chinese.
-
Expanding the horizons of levan: from microbial biosynthesis to applications and advanced detection methods.World J Microbiol Biotechnol. 2024 May 24;40(7):214. doi: 10.1007/s11274-024-04023-w. World J Microbiol Biotechnol. 2024. PMID: 38789837 Review.
Cited by
-
Production and сharacterization of the exopolysaccharide from strain Paenibacillus polymyxa 2020.PLoS One. 2021 Jul 6;16(7):e0253482. doi: 10.1371/journal.pone.0253482. eCollection 2021. PLoS One. 2021. PMID: 34228741 Free PMC article.
-
Levan Production by Suhomyces kilbournensis Using Sugarcane Molasses as a Carbon Source in Submerged Fermentation.Molecules. 2024 Feb 29;29(5):1105. doi: 10.3390/molecules29051105. Molecules. 2024. PMID: 38474615 Free PMC article.
-
Acute Hepatopancreatic Necrosis Disease (AHPND): Virulence, Pathogenesis and Mitigation Strategies in Shrimp Aquaculture.Toxins (Basel). 2021 Jul 27;13(8):524. doi: 10.3390/toxins13080524. Toxins (Basel). 2021. PMID: 34437395 Free PMC article. Review.
-
Efficient production of levan using a recombinant yeast Saccharomyces cerevisiae hypersecreting a bacterial levansucrase.J Ind Microbiol Biotechnol. 2019 Nov;46(11):1611-1620. doi: 10.1007/s10295-019-02206-1. Epub 2019 Jun 22. J Ind Microbiol Biotechnol. 2019. PMID: 31230216
-
Application of microbial extracellular carbohydrate polymeric substances in food and allied industries.3 Biotech. 2020 May;10(5):221. doi: 10.1007/s13205-020-02200-w. Epub 2020 Apr 28. 3 Biotech. 2020. PMID: 32355595 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

