Enhanced production, purification, characterization and mechanism of action of salivaricin 9 lantibiotic produced by Streptococcus salivarius NU10
- PMID: 24147072
- PMCID: PMC3797685
- DOI: 10.1371/journal.pone.0077751
Enhanced production, purification, characterization and mechanism of action of salivaricin 9 lantibiotic produced by Streptococcus salivarius NU10
Abstract
Background: Lantibiotics are small lanthionine-containing bacteriocins produced by lactic acid bacteria. Salivaricin 9 is a newly discovered lantibiotic produced by Streptococcus salivarius. In this study we present the mechanism of action of salivaricin 9 and some of its properties. Also we developed new methods to produce and purify the lantibiotic from strain NU10.
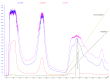
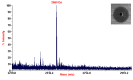
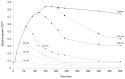
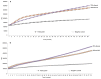
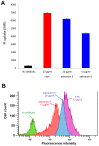
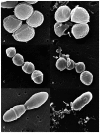
Methodology/principal findings: Salivaricin 9 was found to be auto-regulated when an induction assay was applied and this finding was used to develop a successful salivaricin 9 production system in liquid medium. A combination of XAD-16 and cation exchange chromatography was used to purify the secondary metabolite which was shown to have a molecular weight of approximately 3000 Da by SDS-PAGE. MALDI-TOF MS analysis indicated the presence of salivaricin 9, a 2560 Da lantibiotic. Salivaricin 9 is a bactericidal molecule targeting the cytoplasmic membrane of sensitive cells. The membrane permeabilization assay showed that salivaricin 9 penetrated the cytoplasmic membrane and induced pore formation which resulted in cell death. The morphological changes of test bacterial strains incubated with salivaricin 9 were visualized using Scanning Electron Microscopy which confirmed a pore forming mechanism of inhibition. Salivaricin 9 retained biological stability when exposed to high temperature (90-100°C) and stayed bioactive at pH ranging 2 to 10. When treated with proteinase K or peptidase, salivaricin 9 lost all antimicrobial activity, while it remained active when treated with lyticase, catalase and certain detergents.
Conclusion: The mechanism of antimicrobial action of a newly discovered lantibiotic salivaricin 9 was elucidated in this study. Salivaricin 9 penetrated the cytoplasmic membrane of its targeted cells and induced pore formation. This project has given new insights on lantibiotic peptides produced by S. salivarius isolated from the oral cavities of Malaysian subjects.
Conflict of interest statement
Figures




References
-
- Klocke M, Mundt K, Idler F, Jung S, Backhausen JE (2005) Heterologous expression of enterocin A, a bacteriocin from Enterococcus faecium, fused to a cellulose-binding domain in Escherichia coli results in a functional protein with inhibitory activity against Listeria . Appl Microbiol Biotechnol 67: 532-538. doi:10.1007/s00253-004-1838-5. PubMed: 15660219. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

