Rectified cell migration on saw-like micro-elastically patterned hydrogels with asymmetric gradient ratchet teeth
- PMID: 24147112
- PMCID: PMC3798417
- DOI: 10.1371/journal.pone.0078067
Rectified cell migration on saw-like micro-elastically patterned hydrogels with asymmetric gradient ratchet teeth
Abstract
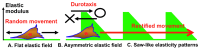
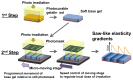
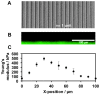
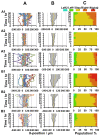
To control cell motility is one of the essential technologies for biomedical engineering. To establish a methodology of the surface design of elastic substrate to control the long-range cell movements, here we report a sophisticated cell culture hydrogel with a micro-elastically patterned surface that allows long-range durotaxis. This hydrogel has a saw-like pattern with asymmetric gradient ratchet teeth, and rectifies random cell movements. Durotaxis only occurs at boundaries in which the gradient strength of elasticity is above a threshold level. Consequently, in gels with unit teeth patterns, durotaxis should only occur at the sides of the teeth in which the gradient strength of elasticity is above this threshold level. Therefore, such gels are expected to support the long-range biased movement of cells via a mechanism similar to the Feynman-Smoluchowski ratchet, i.e., rectified cell migration. The present study verifies this working hypothesis by using photolithographic microelasticity patterning of photocurable gelatin gels. Gels in which each teeth unit was 100-120 µm wide with a ratio of ascending:descending elasticity gradient of 1:2 and a peak elasticity of ca. 100 kPa supported the efficient rectified migration of 3T3 fibroblast cells. In addition, long-range cell migration was most efficient when soft lanes were introduced perpendicular to the saw-like patterns. This study demonstrates that asymmetric elasticity gradient patterning of cell culture gels is a versatile means of manipulating cell motility.
Conflict of interest statement
Figures


Similar articles
-
Elasticity boundary conditions required for cell mechanotaxis on microelastically-patterned gels.Biomaterials. 2011 Apr;32(11):2725-33. doi: 10.1016/j.biomaterials.2011.01.009. Epub 2011 Jan 26. Biomaterials. 2011. PMID: 21276611
-
Time-dependent migratory behaviors in the long-term studies of fibroblast durotaxis on a hydrogel substrate fabricated with a soft band.Langmuir. 2014 Jun 3;30(21):6187-96. doi: 10.1021/la501058j. Epub 2014 May 22. Langmuir. 2014. PMID: 24851722 Free PMC article.
-
Manipulation of cell mechanotaxis by designing curvature of the elasticity boundary on hydrogel matrix.Biomaterials. 2015 Feb;41:45-52. doi: 10.1016/j.biomaterials.2014.11.030. Epub 2014 Dec 5. Biomaterials. 2015. PMID: 25522964
-
Microelastic gradient gelatinous gels to induce cellular mechanotaxis.J Biotechnol. 2008 Jan 20;133(2):225-30. doi: 10.1016/j.jbiotec.2007.08.015. Epub 2007 Aug 14. J Biotechnol. 2008. PMID: 17881075
-
General cellular durotaxis induced with cell-scale heterogeneity of matrix-elasticity.Biomaterials. 2020 Feb;230:119647. doi: 10.1016/j.biomaterials.2019.119647. Epub 2019 Nov 23. Biomaterials. 2020. PMID: 31791844
Cited by
-
A Unidirectional Cell Switching Gate by Engineering Grating Length and Bending Angle.PLoS One. 2016 Jan 28;11(1):e0147801. doi: 10.1371/journal.pone.0147801. eCollection 2016. PLoS One. 2016. PMID: 26821058 Free PMC article.
-
Durotaxis by Human Cancer Cells.Biophys J. 2019 Feb 19;116(4):670-683. doi: 10.1016/j.bpj.2019.01.009. Epub 2019 Jan 12. Biophys J. 2019. PMID: 30709621 Free PMC article.
-
Evaluation of fibroblasts adhesion and proliferation on alginate-gelatin crosslinked hydrogel.PLoS One. 2014 Sep 30;9(9):e107952. doi: 10.1371/journal.pone.0107952. eCollection 2014. PLoS One. 2014. PMID: 25268892 Free PMC article.
-
Mechanical Response of Neural Cells to Physiologically Relevant Stiffness Gradients.Adv Healthc Mater. 2020 Apr;9(8):e1901036. doi: 10.1002/adhm.201901036. Epub 2019 Dec 2. Adv Healthc Mater. 2020. PMID: 31793251 Free PMC article.
-
Interplay among cell migration, shaping, and traction force on a matrix with cell-scale stiffness heterogeneity.Biophys Physicobiol. 2022 Sep 13;19:e190036. doi: 10.2142/biophysico.bppb-v19.0036. eCollection 2022. Biophys Physicobiol. 2022. PMID: 36349327 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

