Glyburide reduces bacterial dissemination in a mouse model of melioidosis
- PMID: 24147174
- PMCID: PMC3798430
- DOI: 10.1371/journal.pntd.0002500
Glyburide reduces bacterial dissemination in a mouse model of melioidosis
Abstract
Background: Burkholderia pseudomallei infection (melioidosis) is an important cause of community-acquired Gram-negative sepsis in Northeast Thailand, where it is associated with a ~40% mortality rate despite antimicrobial chemotherapy. We showed in a previous cohort study that patients taking glyburide ( = glibenclamide) prior to admission have lower mortality and attenuated inflammatory responses compared to patients not taking glyburide. We sought to define the mechanism underlying this observation in a murine model of melioidosis.
Methods: Mice (C57BL/6) with streptozocin-induced diabetes were inoculated with ~6 × 10(2) cfu B. pseudomallei intranasally, then treated with therapeutic ceftazidime (600 mg/kg intraperitoneally twice daily starting 24 h after inoculation) in order to mimic the clinical scenario. Glyburide (50 mg/kg) or vehicle was started 7 d before inoculation and continued until sacrifice. The minimum inhibitory concentration of glyburide for B. pseudomallei was determined by broth microdilution. We also examined the effect of glyburide on interleukin (IL) 1β by bone-marrow-derived macrophages (BMDM).
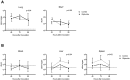
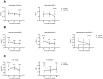
Results: Diabetic mice had increased susceptibility to melioidosis, with increased bacterial dissemination but no effect was seen of diabetes on inflammation compared to non-diabetic controls. Glyburide treatment did not affect glucose levels but was associated with reduced pulmonary cellular influx, reduced bacterial dissemination to both liver and spleen and reduced IL1β production when compared to untreated controls. Other cytokines were not different in glyburide-treated animals. There was no direct effect of glyburide on B. pseudomallei growth in vitro or in vivo. Glyburide directly reduced the secretion of IL1β by BMDMs in a dose-dependent fashion.
Conclusions: Diabetes increases the susceptibility to melioidosis. We further show, for the first time in any model of sepsis, that glyburide acts as an anti-inflammatory agent by reducing IL1β secretion accompanied by diminished cellular influx and reduced bacterial dissemination to distant organs. We found no evidence for a direct effect of glyburide on the bacterium.
Conflict of interest statement
I have read the journal's policy and have the following conflicts to declare: SJP. has received consulting fees from Pfizer. The other authors have no conflicts to report. This does not alter our adherence to all PLOS policies on sharing data and materials.
Figures



References
-
- Wiersinga WJ, Currie BJ, Peacock SJ (2012) Melioidosis. N Engl J Med 367: 1035–1044. - PubMed
-
- Koh GCKW, Maude RR, Schreiber MF, Limmathurotsakul D, Wiersinga WJ, et al. (2011) Glyburide is anti-inflammatory and associated with reduced mortality in melioidosis. Clin Infect Dis 52: 717–725 doi:10.1093/cid/ciq192 - DOI - PMC - PubMed
-
- Department of Health and Human Services (2012) Possession, use, and transfer of select agents and toxins: biennial review. Federal Register 77: 61085–61115. - PubMed
-
- Simpson AJ (2001) Melioidosis: a clinical model for Gram-negative sepsis. J Med Microbiol 50: 657–658. - PubMed
-
- Koh GCKW, Peacock SJ, van der Poll T, Wiersinga WJ (2011) The impact of diabetes on the pathogenesis of sepsis. Eur J Clin Microbiol Infect Dis 31: 379–388 doi:10.1007/s10096-011-1337-4 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

