Raising the pKa limit of "soft" nucleophiles in palladium-catalyzed allylic substitutions: application of diarylmethane pronucleophiles
- PMID: 24147620
- PMCID: PMC3896100
- DOI: 10.1021/ja409511n
Raising the pKa limit of "soft" nucleophiles in palladium-catalyzed allylic substitutions: application of diarylmethane pronucleophiles
Abstract
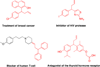
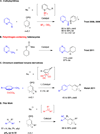
The Tsuji-Trost allylic substitution reaction provides a useful and efficient approach to construct C-C bonds between sp(3)-hybridized carbons. The widely accepted paradigm for classifying the mode of attack of nucleophiles on palladium π-allyl intermediates in the Tsuji-Trost reaction is based on the pKa of the pronucleophile: (1) stabilized or "soft" carbon nucleophiles and heteroatom nucleophiles (e.g., pronucleophiles with pKa's < 25), and (2) unstabilized or "hard" nucleophiles (those from pronucleophiles with pKa's > 25). One of the keys to the continuing development of allylic substitution processes remains broadening the scope of "soft" nucleophiles. Herein we report a general method for the room temperature Pd-catalyzed allylic substitution with diarylmethane derivatives (pKa's up to 32). The synthetic significance of the method is that it provides a rapid access to products containing allylated diarylmethyl motifs. The method is general for a wide range of nucleophiles derived from diarylmethanes and heterocyclic derivatives. A procedure for the Pd-catalyzed allylic substitutions to afford diallylation products with quaternary centers is also described. With triarylmethanes and alkylated diarylmethanes the corresponding allylated products are isolated. We anticipate that the described method will be a valuable complement to the existing arsenal of nucleophiles in Pd-catalyzed allylic substitutions. Mechanistic studies show that the nucleophile derived from diphenylmethane undergoes external attack on π-allyl palladium species under our reaction conditions. This unexpected observation indicates that diarylmethane derivatives behave as "soft" or stabilized nucleophiles. The results of this study indicate that the cutoff between "soft" and "hard" nucleophiles should be raised from a pronucleophile pKa of 25 to at least 32.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

