Molecular grafting onto a stable framework yields novel cyclic peptides for the treatment of multiple sclerosis
- PMID: 24147816
- PMCID: PMC3898541
- DOI: 10.1021/cb400548s
Molecular grafting onto a stable framework yields novel cyclic peptides for the treatment of multiple sclerosis
Abstract
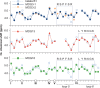
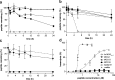
Multiple sclerosis (MS) is an inflammatory disease of the central nervous system (CNS) and is characterized by the destruction of myelin and axons leading to progressive disability. Peptide epitopes from CNS proteins, such as myelin oligodendrocyte glycoprotein (MOG), possess promising immunoregulatory potential for treating MS; however, their instability and poor bioavailability is a major impediment for their use clinically. To overcome this problem, we used molecular grafting to incorporate peptide sequences from the MOG35-55 epitope onto a cyclotide, which is a macrocyclic peptide scaffold that has been shown to be intrinsically stable. Using this approach, we designed novel cyclic peptides that retained the structure and stability of the parent scaffold. One of the grafted peptides, MOG3, displayed potent ability to prevent disease development in a mouse model of MS. These results demonstrate the potential of bioengineered cyclic peptides for the treatment of MS.
Figures



References
-
- Ewing C.; Bernard C. C. (1998) Insights into the aetiology and pathogenesis of multiple sclerosis. Immunol. Cell Biol. 76, 47–54. - PubMed
-
- Bernard C. C.; Kerlero de Rosbo N. (1992) Multiple sclerosis: an autoimmune disease of multifactorial etiology. Curr. Opin. Immunol. 4, 760–765. - PubMed
-
- Onuki M.; Ayers M. M.; Bernard C. C.; Orian J. M. (2001) Axonal degeneration is an early pathological feature in autoimmune-mediated demyelination in mice. Microsc. Res. Tech. 52, 731–739. - PubMed
-
- Ayers M. M.; Hazelwood L. J.; Catmull D. V.; Wang D.; McKormack Q.; Bernard C. C.; Orian J. M. (2004) Early glial responses in murine models of multiple sclerosis. Neurochem. Int. 45, 409–419. - PubMed
-
- Bernard C. C.; Johns T. G.; Slavin A.; Ichikawa M.; Ewing C.; Liu J.; Bettadapura J. (1997) Myelin oligodendrocyte glycoprotein: a novel candidate autoantigen in multiple sclerosis. J. Mol. Med. 75, 77–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

