Metabolic flux between unsaturated and saturated fatty acids is controlled by the FabA:FabB ratio in the fully reconstituted fatty acid biosynthetic pathway of Escherichia coli
- PMID: 24147979
- PMCID: PMC3858588
- DOI: 10.1021/bi401116n
Metabolic flux between unsaturated and saturated fatty acids is controlled by the FabA:FabB ratio in the fully reconstituted fatty acid biosynthetic pathway of Escherichia coli
Abstract
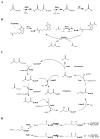
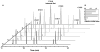
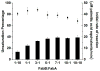
The entire fatty acid biosynthetic pathway of Escherichia coli, starting from the acetyl-CoA carboxylase, has been reconstituted in vitro from 14 purified protein components. Radiotracer analysis verified stoichiometric conversion of acetyl-CoA and NAD(P)H to the free fatty acid product, allowing implementation of a facile spectrophotometric assay for kinetic analysis of this multienzyme system. At steady state, a maximal turnover rate of 0.5 s(-1) was achieved. Under optimal turnover conditions, the predominant products were C16 and C18 saturated as well as monounsaturated fatty acids. The reconstituted system allowed us to quantitatively interrogate the factors that influence metabolic flux toward unsaturated versus saturated fatty acids. In particular, the concentrations of the dehydratase FabA and the β-ketoacyl synthase FabB were found to be crucial for controlling this property. Via changes in these variables, the percentage of unsaturated fatty acid produced could be adjusted between 10 and 50% without significantly affecting the maximal turnover rate of the pathway. Our reconstituted system provides a powerful tool for understanding and engineering rate-limiting and regulatory steps in this complex and practically significant metabolic pathway.
Figures


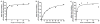

Similar articles
-
Complex binding of the FabR repressor of bacterial unsaturated fatty acid biosynthesis to its cognate promoters.Mol Microbiol. 2011 Apr;80(1):195-218. doi: 10.1111/j.1365-2958.2011.07564.x. Epub 2011 Feb 21. Mol Microbiol. 2011. PMID: 21276098 Free PMC article.
-
Transcriptional regulation of membrane lipid homeostasis in Escherichia coli.J Biol Chem. 2009 Dec 11;284(50):34880-8. doi: 10.1074/jbc.M109.068239. Epub 2009 Oct 23. J Biol Chem. 2009. PMID: 19854834 Free PMC article.
-
Increasing unsaturated fatty acid contents in Escherichia coli by coexpression of three different genes.Appl Microbiol Biotechnol. 2010 Jun;87(1):271-80. doi: 10.1007/s00253-009-2377-x. Epub 2010 Feb 5. Appl Microbiol Biotechnol. 2010. PMID: 20135119
-
The Escherichia coli FadR transcription factor: Too much of a good thing?Mol Microbiol. 2021 Jun;115(6):1080-1085. doi: 10.1111/mmi.14663. Epub 2020 Dec 19. Mol Microbiol. 2021. PMID: 33283913 Free PMC article. Review.
-
The structural biology of type II fatty acid biosynthesis.Annu Rev Biochem. 2005;74:791-831. doi: 10.1146/annurev.biochem.74.082803.133524. Annu Rev Biochem. 2005. PMID: 15952903 Review.
Cited by
-
Synthetic Minimal Cell: Self-Reproduction of the Boundary Layer.ACS Omega. 2019 Mar 31;4(3):5293-5303. doi: 10.1021/acsomega.8b02955. Epub 2019 Mar 13. ACS Omega. 2019. PMID: 30949617 Free PMC article.
-
Analysis of Interdependent Kinetic Controls of Fatty Acid Synthases.ACS Catal. 2018 Dec 7;8(12):11722-11734. doi: 10.1021/acscatal.8b03171. Epub 2018 Oct 31. ACS Catal. 2018. PMID: 30881730 Free PMC article.
-
Exogenous fatty acids inhibit fatty acid synthesis by competing with endogenously generated substrates for phospholipid synthesis in Escherichia coli.FEBS Lett. 2025 Mar;599(5):667-681. doi: 10.1002/1873-3468.15092. Epub 2024 Dec 30. FEBS Lett. 2025. PMID: 39739509 Free PMC article.
-
A temperature-sensitive metabolic valve and a transcriptional feedback loop drive rapid homeoviscous adaptation in Escherichia coli.Nat Commun. 2024 Oct 30;15(1):9386. doi: 10.1038/s41467-024-53677-5. Nat Commun. 2024. PMID: 39477942 Free PMC article.
-
Fatty acid biosynthesis revisited: structure elucidation and metabolic engineering.Mol Biosyst. 2015 Jan;11(1):38-59. doi: 10.1039/c4mb00443d. Epub 2014 Oct 31. Mol Biosyst. 2015. PMID: 25360565 Free PMC article. Review.
References
-
- Liu T, Vora H, Khosla C. Quantitative analysis and engineering of fatty acid biosynthesis in E. coli. Metab Eng. 2010;12:378–386. - PubMed
-
- Neidhardt FC, Curtiss R. Escherichia coli and Salmonella cellular and molecular biology, 2nd ed. ASM Press; Washington, D.C: 1999. p. 1. CD-ROM (4 3/4 in.) +
-
- Tyson KS, McCormick RL United States. Dept. of Energy. Office of Energy Efficiency and Renewable Energy., and National Renewable Energy Laboratory (U.S.) Nrel/Tp 540-40555. 3. National Renewable Energy Laboratory; Golden, CO: 2006. Biodiesel handling and use guidelines; p. iv.p. 61.
-
- Ramos MJ, Fernandez CM, Casas A, Rodriguez L, Perez A. Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour Technol. 2009;100:261–268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

