The cannabinoid CB₂ receptor agonist AM1241 enhances neurogenesis in GFAP/Gp120 transgenic mice displaying deficits in neurogenesis
- PMID: 24148086
- PMCID: PMC3904265
- DOI: 10.1111/bph.12478
The cannabinoid CB₂ receptor agonist AM1241 enhances neurogenesis in GFAP/Gp120 transgenic mice displaying deficits in neurogenesis
Abstract
Background and purpose: HIV-1 glycoprotein Gp120 induces apoptosis in rodent and human neurons in vitro and in vivo. HIV-1/Gp120 is involved in the pathogenesis of HIV-associated dementia (HAD) and inhibits proliferation of adult neural progenitor cells (NPCs) in glial fibrillary acidic protein (GFAP)/Gp120 transgenic (Tg) mice. As cannabinoids exert neuroprotective effects in several model systems, we examined the protective effects of the CB₂ receptor agonist AM1241 on Gp120-mediated insults on neurogenesis.
Experimental approach: We assessed the effects of AM1241 on survival and apoptosis in cultures of human and murine NPCs with immunohistochemical and TUNEL techniques. Neurogenesis in the hippocampus of GFAP/Gp120 transgenic mice in vivo was also assessed by immunohistochemistry.
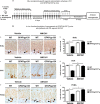
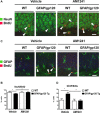
Key results: AM1241 inhibited in vitro Gp120-mediated neurotoxicity and apoptosis of primary human and murine NPCs and increased their survival. AM1241 also promoted differentiation of NPCs to neuronal cells. While GFAP/Gp120 Tg mice exhibited impaired neurogenesis, as indicated by reduction in BrdU⁺ cells and doublecortin⁺ (DCX⁺) cells, and a decrease in cells with proliferating cell nuclear antigen (PCNA), administration of AM1241 to GFAP/Gp120 Tg mice resulted in enhanced in vivo neurogenesis in the hippocampus as indicated by increase in neuroblasts, neuronal cells, BrdU⁺ cells and PCNA⁺ cells. Astrogliosis and gliogenesis were decreased in GFAP/Gp120 Tg mice treated with AM1241, compared with those treated with vehicle.
Conclusions and implications: The CB₂ receptor agonist rescued impaired neurogenesis caused by HIV-1/Gp120 insult. Thus, CB₂ receptor agonists may act as neuroprotective agents, restoring impaired neurogenesis in patients with HAD.
Keywords: CB2 agonist; GFAP/Gp120 transgenic mice; HIV-1 Gp120 protein; neural progenitor cells; neurogenesis.
© 2013 The British Pharmacological Society.
Figures




Similar articles
-
Impaired neurogenesis by HIV-1-Gp120 is rescued by genetic deletion of fatty acid amide hydrolase enzyme.Br J Pharmacol. 2015 Oct;172(19):4603-14. doi: 10.1111/bph.12657. Epub 2015 Aug 21. Br J Pharmacol. 2015. PMID: 24571443 Free PMC article.
-
Glial fibrillary acidic protein-expressing neural progenitors give rise to immature neurons via early intermediate progenitors expressing both glial fibrillary acidic protein and neuronal markers in the adult hippocampus.Neuroscience. 2010 Mar 10;166(1):241-51. doi: 10.1016/j.neuroscience.2009.12.026. Epub 2009 Dec 16. Neuroscience. 2010. PMID: 20026190
-
Adult neurogenic deficits in HIV-1 Tg26 transgenic mice.J Neuroinflammation. 2018 Oct 12;15(1):287. doi: 10.1186/s12974-018-1322-2. J Neuroinflammation. 2018. PMID: 30314515 Free PMC article.
-
Transgenic mice expressing HIV-1 envelope protein gp120 in the brain as an animal model in neuroAIDS research.J Neurovirol. 2018 Apr;24(2):156-167. doi: 10.1007/s13365-017-0584-2. Epub 2017 Oct 26. J Neurovirol. 2018. PMID: 29075998 Free PMC article. Review.
-
HIV-1 and Compromised Adult Neurogenesis: Emerging Evidence for a New Paradigm of HAND Persistence.AIDS Rev. 2019;21(1):11-22. doi: 10.24875/AIDSRev.19000003. AIDS Rev. 2019. PMID: 30899112 Free PMC article. Review.
Cited by
-
Therapeutic potential of EVs loaded with CB2 receptor agonist in spinal cord injury via the Nrf2/HO-1 pathway.Redox Rep. 2024 Dec;29(1):2420572. doi: 10.1080/13510002.2024.2420572. Epub 2024 Oct 28. Redox Rep. 2024. PMID: 39466990 Free PMC article.
-
Potential pharmacological approaches for the treatment of HIV-1 associated neurocognitive disorders.Fluids Barriers CNS. 2020 Jul 10;17(1):42. doi: 10.1186/s12987-020-00204-5. Fluids Barriers CNS. 2020. PMID: 32650790 Free PMC article. Review.
-
Scaling Synapses in the Presence of HIV.Neurochem Res. 2019 Jan;44(1):234-246. doi: 10.1007/s11064-018-2502-2. Epub 2018 Mar 14. Neurochem Res. 2019. PMID: 29541929 Free PMC article. Review.
-
Role of neurotrophic factor alterations in the neurodegenerative process in HIV associated neurocognitive disorders.J Neuroimmune Pharmacol. 2014 Mar;9(2):102-16. doi: 10.1007/s11481-013-9520-2. Epub 2014 Feb 8. J Neuroimmune Pharmacol. 2014. PMID: 24510686 Free PMC article. Review.
-
Reciprocal Influences of HIV and Cannabinoids on the Brain and Cognitive Function.J Neuroimmune Pharmacol. 2020 Dec;15(4):765-779. doi: 10.1007/s11481-020-09921-y. Epub 2020 May 22. J Neuroimmune Pharmacol. 2020. PMID: 32445005 Free PMC article. Review.
References
-
- Aguado T, Monory K, Palazuelos J, Stella N, Cravatt B, Lutz B, et al. The endocannabinoid system drives neural progenitor proliferation. FASEB J. 2005;19:1704–1706. - PubMed
-
- Albright AV, Martin J, O'Connor M, Gonzalez-Scarano F. Interactions between HIV-1 gp120, chemokines, and cultured adult microglial cells. J Neurovirol. 2001;7:196–207. - PubMed
-
- Bari M, Rapino C, Mozetic P, Maccarrone M. The endocannabinoid system in gp120-mediated insults and HIV-associated dementia. Exp Neurol. 2010;224:74–78. - PubMed
-
- Bunnik EM, Euler Z, Welkers MR, Boeser-Nunnink BD, Grijsen ML, Prins JM, et al. Adaptation of HIV-1 envelope gp120 to humoral immunity at a population level. Nat Med. 2010;16:995–997. - PubMed
-
- Cherner M, Ellis RJ, Lazzaretto D, Young C, Mindt MR, Atkinson JH, et al. Effects of HIV-1 infection and aging on neurobehavioral functioning: preliminary findings. AIDS. 2004;18(Suppl 1):S27–S34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

