The TREAT-NMD care and trial site registry: an online registry to facilitate clinical research for neuromuscular diseases
- PMID: 24148153
- PMCID: PMC3819466
- DOI: 10.1186/1750-1172-8-171
The TREAT-NMD care and trial site registry: an online registry to facilitate clinical research for neuromuscular diseases
Abstract
Background: Rare diseases pose many research challenges specific to their scarcity. Advances in potential therapies have made it more important than ever to be able to adequately identify not only patients with particular genotypes (via patient registries) but also the medical professionals who provide care for them at particular specialist centres of expertise and who may be competent to participate in trials. Work within the neuromuscular field provides an example of how this may be achieved.
Methods: This paper describes the development of the TREAT-NMD Care and Trial Site Registry (CTSR), an initiative of an EU-funded Network of Excellence, and its utility in providing an infrastructure for clinical trial feasibility, recruitment, and other studies.
Results: 285 CTSR-registered centres, reporting 35,495 neuromuscular patients, are described alongside an analysis of their provision for DMD. Site characteristics vary by country: the average number of DMD patients seen per site in the United States (96) is more than in Germany (25), and paediatric/adult breakdown is also markedly distinct. Over 70% of sites have previous trial experience, with a majority including a Clinical Trials Unit. Most sites also have MLPA diagnostic capability and access to a range of medical specialists. However, in the three countries reporting most sites (US, the UK and Germany), few had access to all core DMD specialists internally. Over 60% of sites did not report any form of transition arrangement.
Conclusions: Registries of care and trial sites have significant utility for research into rare conditions such as neuromuscular diseases, demonstrated by the significant engagement by industry and other researchers with the CTSR. We suggest that this approach may be applicable to other fields needing to identify centres of expertise with the potential to carry out clinical research and engage in clinical trials. Such registries also lend themselves to the developing context of European Reference Networks (ERNs), which seek to build networks of centres of expertise which fit specific criteria, and which may themselves aid the sustainability of such registries. This is particularly the case given the utility of registries such as the CTSR in enabling networks of best-practice care centres.
Figures

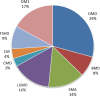

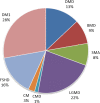

References
-
- COM(2008) 679 Final: Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions on Rare Diseases: Europe’s Challenges. [ http://ec.europa.eu/health/ph_threats/non_com/docs/rare_com_en.pdf]
-
- TREAT-NMD. [ http://www.treat-nmd.eu/]
-
- Taruscio D, Gainotti S, Vittozzi L, Bianchi F, Ensini M, Posada M. EPIRARE survey on activities and needs of rare disease registries in the European Union. Orphanet J Rare Dis. 2012;7:A22. doi: 10.1186/1750-1172-7-S2-A22. - DOI
-
- Rare disease registries in Europe, January 2013, Orphanet report series. [ http://www.orpha.net/orphacom/cahiers/docs/GB/Registries.pdf]
-
- The Parkinson Study Group. [ http://www.parkinson-study-group.org]
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

