Impact of a population-based HPV vaccination program on cervical abnormalities: a data linkage study
- PMID: 24148310
- PMCID: PMC4015688
- DOI: 10.1186/1741-7015-11-227
Impact of a population-based HPV vaccination program on cervical abnormalities: a data linkage study
Abstract
Background: Australia was one of the first countries to introduce a publicly funded national human papillomavirus (HPV) vaccination program that commenced in April 2007, using the quadrivalent HPV vaccine targeting 12- to 13-year-old girls on an ongoing basis. Two-year catch-up programs were offered to 14- to 17- year-old girls in schools and 18- to 26-year-old women in community-based settings. We present data from the school-based program on population-level vaccine effectiveness against cervical abnormalities in Victoria, Australia.
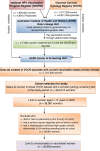
Methods: Data for women age-eligible for the HPV vaccination program were linked between the Victorian Cervical Cytology Registry and the National HPV Vaccination Program Register to create a cohort of screening women who were either vaccinated or unvaccinated. Entry into the cohort was 1 April 2007 or at first Pap test for women not already screening. Vaccine effectiveness (VE) and hazard ratios (HR) for cervical abnormalities by vaccination status between 1 April 2007 and 31 December 2011 were calculated using proportional hazards regression.
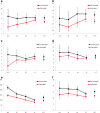
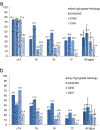
Results: The study included 14,085 unvaccinated and 24,871 vaccinated women attending screening who were eligible for vaccination at school, 85.0% of whom had received three doses. Detection rates of histologically confirmed high-grade (HG) cervical abnormalities and high-grade cytology (HGC) were significantly lower for vaccinated women (any dose) (HG 4.8 per 1,000 person-years, HGC 11.9 per 1,000 person-years) compared with unvaccinated women (HG 6.4 per 1,000 person-years, HGC 15.3 per 1,000 person-years) HR 0.72 (95% CI 0.58 to 0.91) and HR 0.75 (95% CI 0.65 to 0.87), respectively. The HR for low-grade (LG) cytological abnormalities was 0.76 (95% CI 0.72 to 0.80). VE adjusted a priori for age at first screening, socioeconomic status and remoteness index, for women who were completely vaccinated, was greatest for CIN3+/AIS at 47.5% (95% CI 22.7 to 64.4) and 36.4% (95% CI 9.8 to 55.1) for women who received any dose of vaccine, and was negatively associated with age. For women who received only one or two doses of vaccine, HRs for HG histology were not significantly different from 1.0, although the number of outcomes was small.
Conclusion: A population-based HPV vaccination program in schools significantly reduced cervical abnormalities for vaccinated women within five years of implementation, with the greatest vaccine effectiveness observed for the youngest women.
Figures
References
-
- Li N, Franceschi S, Howell-Jones R, Snijders PJ, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: variation by geographical region, histological type and year of publication. Int J Cancer. 2010;11:927–935. - PubMed
-
- The Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. New Engl J Med. 2007;11:1915–1927. - PubMed
-
- Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC, Skinner SR, Hedrick J, Jaisamrarn U, Limson G, Garland S, Szarewski A, Romanowski B, Aoki FY, Schwarz TF, Poppe WA, Bosch FX, Jenkins D, Hardt K, Zahaf T, Descamps D, Struyf F, Lehtinen M, Dubin G. HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;11:301–314. doi: 10.1016/S0140-6736(09)61248-4. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

