Intrahypothalamic pituitary adenylate cyclase-activating polypeptide regulates energy balance via site-specific actions on feeding and metabolism
- PMID: 24148346
- PMCID: PMC3882380
- DOI: 10.1152/ajpendo.00293.2013
Intrahypothalamic pituitary adenylate cyclase-activating polypeptide regulates energy balance via site-specific actions on feeding and metabolism
Abstract
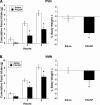
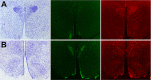
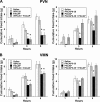
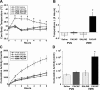
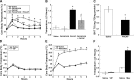
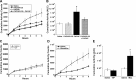
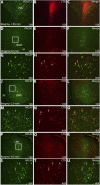
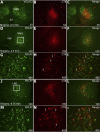
Numerous studies have demonstrated that both the hypothalamic paraventricular nuclei (PVN) and ventromedial nuclei (VMN) regulate energy homeostasis through behavioral and metabolic mechanisms. Receptors for pituitary adenylate cyclase-activating polypeptide (PACAP) are abundantly expressed in these nuclei, suggesting PACAP may be critical for the regulation of feeding behavior and body weight. To characterize the unique behavioral and physiological responses attributed to select hypothalamic cell groups, PACAP was site-specifically injected into the PVN or VMN. Overall food intake was significantly reduced by PACAP at both sites; however, meal pattern analysis revealed that only injections into the PVN produced significant reductions in meal size, duration, and total time spent eating. PACAP-mediated hypophagia in both the PVN and VMN was abolished by PAC1R antagonism, whereas pretreatment with a VPACR antagonist had no effect. PACAP injections into the VMN produced unique changes in metabolic parameters, including significant increases in core body temperature and spontaneous locomotor activity that was PAC1R dependent whereas, PVN injections of PACAP had no effect. Finally, PACAP-containing afferents were identified using the neuronal tracer cholera toxin subunit B (CTB) injected unilaterally into the PVN or VMN. CTB signal from PVN injections was colocalized with PACAP mRNA in the medial anterior bed nucleus of the stria terminalis, VMN, and lateral parabrachial nucleus (LPB), whereas CTB signal from VMN injections was highly colocalized with PACAP mRNA in the medial amygdala and LPB. These brain regions are known to influence energy homeostasis perhaps, in part, through PACAP projections to the PVN and VMN.
Keywords: activity; feeding; hypothalamus; rat; temperature.
Figures

Similar articles
-
The PACAP Paradox: Dynamic and Surprisingly Pleiotropic Actions in the Central Regulation of Energy Homeostasis.Front Endocrinol (Lausanne). 2022 Jun 1;13:877647. doi: 10.3389/fendo.2022.877647. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35721722 Free PMC article. Review.
-
Stimulation of the hypothalamic ventromedial nuclei by pituitary adenylate cyclase-activating polypeptide induces hypophagia and thermogenesis.Am J Physiol Regul Integr Comp Physiol. 2011 Dec;301(6):R1625-34. doi: 10.1152/ajpregu.00334.2011. Epub 2011 Sep 28. Am J Physiol Regul Integr Comp Physiol. 2011. PMID: 21957159 Free PMC article.
-
Pituitary adenylate cyclase-activating polypeptide receptor activation in the hypothalamus recruits unique signaling pathways involved in energy homeostasis.Am J Physiol Endocrinol Metab. 2022 Mar 1;322(3):E199-E210. doi: 10.1152/ajpendo.00320.2021. Epub 2022 Jan 10. Am J Physiol Endocrinol Metab. 2022. PMID: 35001657 Free PMC article.
-
Hypothalamic and brainstem sources of pituitary adenylate cyclase-activating polypeptide nerve fibers innervating the hypothalamic paraventricular nucleus in the rat.J Comp Neurol. 2007 Feb 1;500(4):761-76. doi: 10.1002/cne.21212. J Comp Neurol. 2007. PMID: 17154257 Free PMC article.
-
Hypothalamic PACAP/PAC1R Involvement in Feeding and Body Weight Regulation.Endocrinology. 2023 Mar 13;164(5):bqad044. doi: 10.1210/endocr/bqad044. Endocrinology. 2023. PMID: 36917637 Review.
Cited by
-
Adipose Tissue Expression of PACAP, VIP, and Their Receptors in Response to Cold Stress.J Mol Neurosci. 2019 Jul;68(3):427-438. doi: 10.1007/s12031-018-1099-x. Epub 2018 Jul 7. J Mol Neurosci. 2019. PMID: 29982965 Free PMC article.
-
Pleiotropic pituitary adenylate cyclase-activating polypeptide (PACAP): Novel insights into the role of PACAP in eating and drug intake.Brain Res. 2020 Feb 15;1729:146626. doi: 10.1016/j.brainres.2019.146626. Epub 2019 Dec 26. Brain Res. 2020. PMID: 31883848 Free PMC article. Review.
-
Synaptic plasticity and the role of astrocytes in central metabolic circuits.WIREs Mech Dis. 2024 Jan-Feb;16(1):e1632. doi: 10.1002/wsbm.1632. Epub 2023 Oct 13. WIREs Mech Dis. 2024. PMID: 37833830 Free PMC article. Review.
-
PACAP in the BNST produces anorexia and weight loss in male and female rats.Neuropsychopharmacology. 2014 Jun;39(7):1614-23. doi: 10.1038/npp.2014.8. Epub 2014 Jan 17. Neuropsychopharmacology. 2014. PMID: 24434744 Free PMC article.
-
The PACAP Paradox: Dynamic and Surprisingly Pleiotropic Actions in the Central Regulation of Energy Homeostasis.Front Endocrinol (Lausanne). 2022 Jun 1;13:877647. doi: 10.3389/fendo.2022.877647. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35721722 Free PMC article. Review.
References
-
- Agarwal A, Halvorson LM, Legradi G. Pituitary adenylate cyclase-activating polypeptide (PACAP) mimics neuroendocrine and behavioral manifestations of stress: Evidence for PKA-mediated expression of the corticotropin-releasing hormone (CRH) gene. Brain Res Mol Brain Res 138: 45–57, 2005 - PMC - PubMed
-
- Amir S. Intra-ventromedial hypothalamic injection of glutamate stimulates brown adipose tissue thermogenesis in the rat. Brain Res 511: 341–344, 1990 - PubMed
-
- Amir S. Stimulation of the paraventricular nucleus with glutamate activates interscapular brown adipose tissue thermogenesis in rats. Brain Res 508: 152–155, 1990 - PubMed
-
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123: 493–505, 2005 - PubMed
-
- Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 276: R1569–R1578, 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

