Extensive conversion of hepatic biliary epithelial cells to hepatocytes after near total loss of hepatocytes in zebrafish
- PMID: 24148620
- PMCID: PMC3943869
- DOI: 10.1053/j.gastro.2013.10.019
Extensive conversion of hepatic biliary epithelial cells to hepatocytes after near total loss of hepatocytes in zebrafish
Abstract
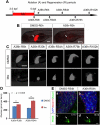
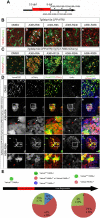
Background & aims: Biliary epithelial cells (BECs) are considered to be a source of regenerating hepatocytes when hepatocyte proliferation is compromised. However, there is still controversy about the extent to which BECs can contribute to the regenerating hepatocyte population, and thereby to liver recovery. To investigate this issue, we established a zebrafish model of liver regeneration in which the extent of hepatocyte ablation can be controlled.
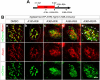
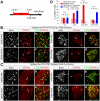
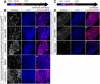
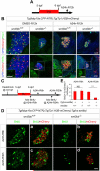
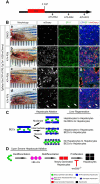
Methods: Hepatocytes were depleted by administration of metronidazole to Tg(fabp10a:CFP-NTR) animals. We traced the origin of regenerating hepatocytes using short-term lineage-tracing experiments, as well as the inducible Cre/loxP system; specifically, we utilized both a BEC tracer line Tg(Tp1:CreER(T2)) and a hepatocyte tracer line Tg(fabp10a:CreER(T2)). We also examined BEC and hepatocyte proliferation and liver marker gene expression during liver regeneration.
Results: BECs gave rise to most of the regenerating hepatocytes in larval and adult zebrafish after severe hepatocyte depletion. After hepatocyte loss, BECs proliferated as they dedifferentiated into hepatoblast-like cells; they subsequently differentiated into highly proliferative hepatocytes that restored the liver mass. This process was impaired in zebrafish wnt2bb mutants; in these animals, hepatocytes regenerated but their proliferation was greatly reduced.
Conclusions: BECs contribute to regenerating hepatocytes after substantial hepatocyte depletion in zebrafish, thereby leading to recovery from severe liver damage.
Keywords: Dedifferentiation; Liver Regeneration; Oval Cells; Stem Cells.
Copyright © 2014 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures
Comment in
-
Biliary cells to the rescue of Prometheus.Gastroenterology. 2014 Mar;146(3):611-4. doi: 10.1053/j.gastro.2014.01.039. Epub 2014 Jan 23. Gastroenterology. 2014. PMID: 24462990 No abstract available.
-
Conversion of hepatic biliary cells to hepatocytes in zebrafish.Gastroenterology. 2014 Mar;146(3):597-8. Gastroenterology. 2014. PMID: 24701641 No abstract available.
References
-
- Fausto N, Campbell JS. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mechanisms of Development. 2003;120:117–130. - PubMed
-
- Okabe M, Tsukahara Y, Tanaka M, et al. Potential hepatic stem cells reside in EpCAM(+) cells of normal and injured mouse liver. Development. 2009;136:1951–1960. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

