Adult ciliary epithelial stem cells generate functional neurons and differentiate into both early and late born retinal neurons under non-cell autonomous influences
- PMID: 24148749
- PMCID: PMC3856605
- DOI: 10.1186/1471-2202-14-130
Adult ciliary epithelial stem cells generate functional neurons and differentiate into both early and late born retinal neurons under non-cell autonomous influences
Abstract
Background: The neural stem cells discovered in the adult ciliary epithelium (CE) in higher vertebrates have emerged as an accessible source of retinal progenitors; these cells can self-renew and possess retinal potential. However, recent studies have cast doubt as to whether these cells could generate functional neurons and differentiate along the retinal lineage. Here, we have systematically examined the pan neural and retinal potential of CE stem cells.
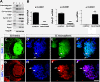
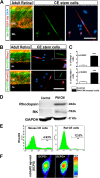
Results: Molecular and cellular analysis was carried out to examine the plasticity of CE stem cells, obtained from mice expressing green fluorescent protein (GFP) under the influence of the promoter of the rod photoreceptor-specific gene, Nrl, using the neurospheres assay. Differentiation was induced by specific culture conditions and evaluated by both transcripts and protein levels of lineage-specific regulators and markers. Temporal pattern of their levels were examined to determine the expression of genes and proteins underlying the regulatory hierarchy of cells specific differentiation in vitro. Functional attributes of differentiation were examined by the presence of current profiles and pharmacological mobilization of intracellular calcium using whole cell recordings and Fura-based calcium imaging, respectively. We demonstrate that stem cells in adult CE not only have the capacity to generate functional neurons, acquiring the expression of sodium and potassium channels, but also respond to specific cues in culture and preferentially differentiate along the lineages of retinal ganglion cells (RGCs) and rod photoreceptors, the early and late born retinal neurons, respectively. The retinal differentiation of CE stem cells was characterized by the temporal acquisition of the expression of the regulators of RGCs and rod photoreceptors, followed by the display of cell type-specific mature markers and mobilization of intracellular calcium.
Conclusions: Our study demonstrates the bonafide retinal potential of adult CE stem cells and suggests that their plasticity could be harnessed for clinical purposes once barriers associated with any lineage conversion, i.e., low efficiency and fidelity is overcome through the identification of conducive culture conditions.
Figures





Similar articles
-
Differences between the neurogenic and proliferative abilities of Müller glia with stem cell characteristics and the ciliary epithelium from the adult human eye.Exp Eye Res. 2011 Dec;93(6):852-61. doi: 10.1016/j.exer.2011.09.015. Epub 2011 Oct 5. Exp Eye Res. 2011. PMID: 21989110 Free PMC article.
-
Retinal properties and potential of the adult mammalian ciliary epithelium stem cells.Vision Res. 2005 Jun;45(13):1653-66. doi: 10.1016/j.visres.2004.12.017. Vision Res. 2005. PMID: 15792841
-
Adult ciliary epithelial cells, previously identified as retinal stem cells with potential for retinal repair, fail to differentiate into new rod photoreceptors.Stem Cells. 2010 Jun;28(6):1048-59. doi: 10.1002/stem.423. Stem Cells. 2010. PMID: 20506130
-
Retinal Stem Cell 'Retirement Plans': Growth, Regulation and Species Adaptations in the Retinal Ciliary Marginal Zone.Int J Mol Sci. 2021 Jun 18;22(12):6528. doi: 10.3390/ijms22126528. Int J Mol Sci. 2021. PMID: 34207050 Free PMC article. Review.
-
Neurogenic potential of stem/progenitor-like cells in the adult mammalian eye.Prog Retin Eye Res. 2012 May;31(3):213-42. doi: 10.1016/j.preteyeres.2012.02.001. Epub 2012 Feb 13. Prog Retin Eye Res. 2012. PMID: 22353284 Review.
Cited by
-
Using Electrical Stimulation to Enhance the Efficacy of Cell Transplantation Therapies for Neurodegenerative Retinal Diseases: Concepts, Challenges, and Future Perspectives.Cell Transplant. 2017 Jun 9;26(6):949-965. doi: 10.3727/096368917X694877. Epub 2017 Feb 3. Cell Transplant. 2017. PMID: 28155808 Free PMC article. Review.
-
Photoreceptor cells with profound structural deficits can support useful vision in mice.Invest Ophthalmol Vis Sci. 2014 Mar 25;55(3):1859-66. doi: 10.1167/iovs.13-13661. Invest Ophthalmol Vis Sci. 2014. PMID: 24569582 Free PMC article.
-
Activation of adult mammalian retinal stem cells in vivo via antagonism of BMP and sFRP2.Stem Cell Res Ther. 2021 Oct 30;12(1):560. doi: 10.1186/s13287-021-02630-0. Stem Cell Res Ther. 2021. PMID: 34717744 Free PMC article.
-
Biofabrication of Artificial Stem Cell Niches in the Anterior Ocular Segment.Bioengineering (Basel). 2021 Sep 30;8(10):135. doi: 10.3390/bioengineering8100135. Bioengineering (Basel). 2021. PMID: 34677208 Free PMC article. Review.
-
Platelet-Activating Factor Receptor (PAFR) Regulates Retinal Progenitor/Stem Cells Profile in Ciliary Epithelium Cells.Int J Mol Sci. 2024 Mar 7;25(6):3084. doi: 10.3390/ijms25063084. Int J Mol Sci. 2024. PMID: 38542059 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

