Development and feasibility testing of decision support for patients who are candidates for a prophylactic implantable defibrillator: a study protocol for a pilot randomized controlled trial
- PMID: 24148851
- PMCID: PMC4015905
- DOI: 10.1186/1745-6215-14-346
Development and feasibility testing of decision support for patients who are candidates for a prophylactic implantable defibrillator: a study protocol for a pilot randomized controlled trial
Abstract
Background: Patients, identified to be at risk for but who have never experienced a potentially lethal cardiac arrhythmia, have the option of receiving an implantable cardioverter defibrillator (ICD) as prophylaxis against sudden cardiac death - a primary prevention indication. In Canada, there is no clear framework to support patients' decision-making for these devices. Decision support, using a decision aid, could moderate treatment-related uncertainty and prepare patients to make well-informed decisions. Patient decision aids provide information on treatment options, risks, and benefits, to help patients clarify their values for outcomes of treatment options. The objectives of this research are: 1) develop a decision aid, 2) evaluate the decision aid, and 3) determine the feasibility of conducting a trial.
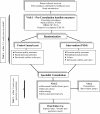
Methods/design: A development panel comprised of the core investigative team, health service researchers, decision science experts, cardiovascular healthcare practitioners, and ICD patient representatives will collaborate to provide input on the content and format of the aid. To generate probabilities to include in the aid, we will synthesize primary prevention ICD evidence. To obtain anonymous input about the facts and content, we will employ a modified Delphi process. To evaluate the draft decision aid will invite ICD patients and their families (n = 30) to rate its acceptability. After we evaluate the aid, to determine the feasibility, we will conduct a feasibility pilot randomized controlled trial (RCT) in new ICD candidates (n = 80). Participants will be randomized to receive a decision aid prior to specialist consultation versus usual care. Results from the pilot RCT will determine the feasibility of research processes; inform sample size calculation, measure decision quality (knowledge, values, decision conflict) and the influence of health related quality of life on decision-making.
Discussion: Our study seeks to develop a decision aid, for patients offered their first ICD for prophylaxis against sudden cardiac death. This paper outlines the background and methods of a pilot randomized trial which will inform a larger multicenter trial. Ultimately, decision support prior to specialist consultation could enhance the decision-making process between patients, physicians, and families, associated with life-prolonging medical devices like the ICD.
Trial registration: ClinicalTrials.gov: NCT01876173.
Figures
References
-
- Tang AS, Ross H, Simpson CS, Mitchell LB, Dorian P, Goeree R. et al.Canadian cardiovascular society/Canadian heart rhythm society position paper on implantable cardioverter defibrillator use in Canada. Can J Cardiol. 2005;14:11A–18A. - PubMed
-
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De SG. et al.Heart disease and stroke statistics - 2010 update: a report from the American heart association.[erratum appears in circulation. 30 March 2010;121(12):e260 note: Stafford, Randall [corrected to Roger, Veronique L]] Circulation. 2010;14:e46–e215. - PubMed
-
- Connolly SJ, Hallstrom AP, Cappato R, Schron EB, Kuck KH, Zipes DP. et al.Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. Antiarrhythmics versus implantable defibrillator study. Cardiac arrest study Hamburg. Canadian implantable defibrillator study. Eur Heart J. 2000;14:2071–2078. doi: 10.1053/euhj.2000.2476. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical