Zebrafish sex: a complicated affair
- PMID: 24148942
- PMCID: PMC3954038
- DOI: 10.1093/bfgp/elt041
Zebrafish sex: a complicated affair
Abstract
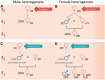
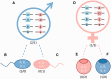
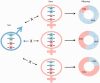
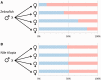
In this review, we provide a detailed overview of studies on the elusive sex determination (SD) and gonad differentiation mechanisms of zebrafish (Danio rerio). We show that the data obtained from most studies are compatible with polygenic sex determination (PSD), where the decision is made by the allelic combinations of several loci. These loci are typically dispersed throughout the genome, but in some teleost species a few of them might be located on a preferential pair of (sex) chromosomes. The PSD system has a much higher level of variation of SD genotypes both at the level of gametes and the sexual genotype of individuals, than that of the chromosomal sex determination systems. The early sexual development of zebrafish males is a complicated process, as they first develop a 'juvenile ovary', that later undergoes a transformation to give way to a testis. To date, three major developmental pathways were shown to be involved with gonad differentiation through the modulation of programmed cell death. In our opinion, there are more pathways participating in the regulation of zebrafish gonad differentiation/transformation. Introduction of additional powerful large-scale genomic approaches into the analysis of zebrafish reproduction will result in further deepening of our knowledge as well as identification of additional pathways and genes associated with these processes in the near future.
Keywords: Danio rerio; fish; gonad differentiation; polygenic sex determination; sex chromosome; teleost.
Figures
Similar articles
-
Long and winding roads: testis differentiation in zebrafish.Mol Cell Endocrinol. 2009 Nov 27;312(1-2):35-41. doi: 10.1016/j.mce.2009.04.014. Epub 2009 May 5. Mol Cell Endocrinol. 2009. PMID: 19422878 Review.
-
Genetic mechanism underlying sexual plasticity and its association with colour patterning in zebrafish (Danio rerio).BMC Genomics. 2019 May 6;20(1):341. doi: 10.1186/s12864-019-5722-1. BMC Genomics. 2019. PMID: 31060508 Free PMC article.
-
Two phases of gonadal sex differentiation in zebrafish with ZZ/ZW sex determination system.Gen Comp Endocrinol. 2024 Nov 1;358:114613. doi: 10.1016/j.ygcen.2024.114613. Epub 2024 Sep 19. Gen Comp Endocrinol. 2024. PMID: 39303945
-
Gonad differentiation in zebrafish is regulated by the canonical Wnt signaling pathway.Biol Reprod. 2014 Feb 27;90(2):45. doi: 10.1095/biolreprod.113.110874. Print 2014 Feb. Biol Reprod. 2014. PMID: 24174574
-
Finding clues to the riddle of sex determination in zebrafish.J Biosci. 2016 Mar;41(1):145-55. doi: 10.1007/s12038-016-9593-1. J Biosci. 2016. PMID: 26949096 Review.
Cited by
-
Drug repurposing for neurodegenerative diseases using Zebrafish behavioral profiles.Biomed Pharmacother. 2024 Feb;171:116096. doi: 10.1016/j.biopha.2023.116096. Epub 2024 Jan 6. Biomed Pharmacother. 2024. PMID: 38185043 Free PMC article.
-
Zebrafish Larvae Position Tracker (Z-LaP Tracker): a high-throughput deep-learning behavioral approach for the identification of calcineurin pathway-modulating drugs using zebrafish larvae.Sci Rep. 2023 Feb 23;13(1):3174. doi: 10.1038/s41598-023-30303-w. Sci Rep. 2023. PMID: 36823315 Free PMC article.
-
Integrated Analysis of Differential Expression Profiles of miRNA and mRNA in Gonads of Scatophagus argus Provides New Insights into Sexually Biased Gene Expression.Animals (Basel). 2025 May 27;15(11):1564. doi: 10.3390/ani15111564. Animals (Basel). 2025. PMID: 40509029 Free PMC article.
-
Clemizole and trazodone are effective antiseizure treatments in a zebrafish model of STXBP1 disorder.Epilepsia Open. 2022 Sep;7(3):504-511. doi: 10.1002/epi4.12604. Epub 2022 May 17. Epilepsia Open. 2022. PMID: 35451230 Free PMC article.
-
Zebrafish: Housing and husbandry recommendations.Lab Anim. 2020 Jun;54(3):213-224. doi: 10.1177/0023677219869037. Epub 2019 Sep 11. Lab Anim. 2020. PMID: 31510859 Free PMC article.
References
-
- Nelson JS. Fishes of the World. 4th. Hoboken, NJ, USA: John Wiley & Sons, Inc; 2007.
-
- Lévêque C, Oberdorff T, Paugy D, et al. Global diversity of fish (Pisces) in freshwater. Hydrobiologia. 2008;595:545–67.
-
- Devlin RH, Nagahama Y. Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture. 2002;208:191–364.
-
- Penman DJ, Piferrer F. Fish gonadogenesis. Part I: Genetic and environmental mechanisms of sex determination. Rev Fish Sci. 2008;16:16–34.
-
- Graves JAM. Weird animal genomes and the evolution of vertebrate sex and sex chromosomes. Annu Rev Genet. 2008;42:565–86. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

