High ALK mRNA expression has a negative prognostic significance in rhabdomyosarcoma
- PMID: 24149177
- PMCID: PMC3859940
- DOI: 10.1038/bjc.2013.653
High ALK mRNA expression has a negative prognostic significance in rhabdomyosarcoma
Abstract
Background: Anaplastic lymphoma kinase (ALK) is a receptor tyrosine kinase aberrantly expressed in cancer, but its clinical and functional importance remain controversial. Mutation or amplification of ALK, as well as its expression levels assessed by conventional immunohistochemistry methods, has been linked to prognosis in cancer, although with potential bias because of the semi-quantitative approaches. Herein, we measured ALK mRNA expression in rhabdomyosarcoma (RMS) and determined its clinical impact on patients' stratification and outcome.
Methods: Specimens were obtained from RMS patients and cell lines, and ALK expression was analysed by quantitative RT-PCR, western blotting, IHC, and copy number analysis.
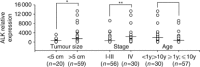
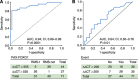
Results: High ALK mRNA expression was detected in the vast majority of PAX3/7-FOXO1-positive tumours, whereas PAX3/7-FOXO1-negative RMS displayed considerably lower amounts of both mRNA and protein. Notably, ALK mRNA distinguished unfavourable PAX3/7-FOXO1-positive tumours from PAX3/7-FOXO1-negative RMS (P<0.0001), and also correlated with larger tumour size (P<0.05) and advanced clinical stage (P<0.01), independently of fusion gene status. High ALK mRNA levels were of prognostic relevance by Cox univariate regression analysis and correlated with increased risk of relapse (P=0.001) and survival (P=0.01), whereas by multivariate analysis elevated ALK mRNA expression resulted a negative prognostic marker when clinical stage was not included.
Conclusion: Quantitative assessment of ALK mRNA expression helps to improve risk stratification of RMS patients and identifies tumours with adverse biological characteristics and aggressive behaviour.
Figures



Similar articles
-
Anaplastic lymphoma kinase aberrations in rhabdomyosarcoma: clinical and prognostic implications.J Clin Oncol. 2012 Jan 20;30(3):308-15. doi: 10.1200/JCO.2011.37.8588. Epub 2011 Dec 19. J Clin Oncol. 2012. PMID: 22184391
-
Anaplastic lymphoma kinase aberrations correlate with metastatic features in pediatric rhabdomyosarcoma.Oncotarget. 2016 Sep 13;7(37):58903-58914. doi: 10.18632/oncotarget.10368. Oncotarget. 2016. PMID: 27385213 Free PMC article.
-
Prognostic implications of anaplastic lymphoma kinase gene aberrations in rhabdomyosarcoma; an immunohistochemical and fluorescence in situ hybridisation study.J Clin Pathol. 2014 Jan;67(1):33-9. doi: 10.1136/jclinpath-2013-201655. Epub 2013 Aug 6. J Clin Pathol. 2014. PMID: 23922356
-
Genetic Characterization, Current Model Systems and Prognostic Stratification in PAX Fusion-Negative vs. PAX Fusion-Positive Rhabdomyosarcoma.Genes (Basel). 2021 Sep 25;12(10):1500. doi: 10.3390/genes12101500. Genes (Basel). 2021. PMID: 34680895 Free PMC article. Review.
-
Pathobiology of NPM-ALK and variant fusion genes in anaplastic large cell lymphoma and other lymphomas.Leukemia. 2000 Sep;14(9):1533-59. doi: 10.1038/sj.leu.2401878. Leukemia. 2000. PMID: 10994999 Review.
Cited by
-
MicroRNA-27a Contributes to Rhabdomyosarcoma Cell Proliferation by Suppressing RARA and RXRA.PLoS One. 2015 Apr 27;10(4):e0125171. doi: 10.1371/journal.pone.0125171. eCollection 2015. PLoS One. 2015. PMID: 25915942 Free PMC article.
-
A humanized anaplastic lymphoma kinase (ALK)-directed antibody-drug conjugate with pyrrolobenzodiazepine payload demonstrates efficacy in ALK-expressing cancers.Nat Commun. 2025 Aug 15;16(1):7578. doi: 10.1038/s41467-025-62979-1. Nat Commun. 2025. PMID: 40813394 Free PMC article.
-
Molecular Targets in Alveolar Rhabdomyosarcoma: A Narrative Review of Progress and Pitfalls.Int J Mol Sci. 2025 May 28;26(11):5204. doi: 10.3390/ijms26115204. Int J Mol Sci. 2025. PMID: 40508013 Free PMC article. Review.
-
Crizotinib-induced antitumour activity in human alveolar rhabdomyosarcoma cells is not solely dependent on ALK and MET inhibition.J Exp Clin Cancer Res. 2015 Oct 6;34:112. doi: 10.1186/s13046-015-0228-4. J Exp Clin Cancer Res. 2015. PMID: 26445453 Free PMC article.
-
Targeting ALK in pediatric RMS does not induce antitumor activity in vivo.Cancer Chemother Pharmacol. 2018 Aug;82(2):251-263. doi: 10.1007/s00280-018-3615-7. Epub 2018 May 31. Cancer Chemother Pharmacol. 2018. PMID: 29855693 Free PMC article.
References
-
- Barr FG, Qualman SJ, Macris MH, Melnyk N, Lawlor ER, Strzelecki DM, Triche TJ, Bridge JA, Sorensen PH. Genetic heterogeneity in the alveolar rhabdomyosarcoma subset without typical gene fusions. Cancer Res. 2002;62:4704–4710. - PubMed
-
- Barreca A, Lasorsa E, Riera L, Machiorlatti R, Piva R, Ponzoni M, Kwee I, Bertoni F, Piccaluga PP, Pileri SA, Inghirami G. Anaplastic lymphoma kinase in human cancer. J Mol Endocrinol. 2011;47:R11–R23. - PubMed
-
- Bonvini P, Gastaldi T, Falini B, Rosolen A. Nucleophosmin-anaplastic lymphoma kinase (NPM-ALK), a novel Hsp90-client tyrosine kinase: down-regulation of NPM-ALK expression and tyrosine phosphorylation in ALK(+) CD30(+) lymphoma cells by the Hsp90 antagonist 17-allylamino,17-demethoxygeldanamycin. Cancer Res. 2002;62:1559–1566. - PubMed
-
- Corao DA, Biegel JA, Coffin CM, Barr FG, Wainwright LM, Ernst LM, Choi JK, Zhang PJ, Pawel BR. ALK expression in rhabdomyosarcomas: correlation with histologic subtype and fusion status. Pediatr Dev Pathol. 2009;12:275–283. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

