Infectious bronchitis virus generates spherules from zippered endoplasmic reticulum membranes
- PMID: 24149513
- PMCID: PMC3812713
- DOI: 10.1128/mBio.00801-13
Infectious bronchitis virus generates spherules from zippered endoplasmic reticulum membranes
Abstract
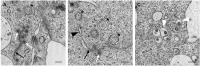
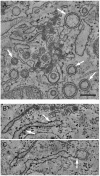
Replication of positive-sense RNA viruses is associated with the rearrangement of cellular membranes. Previous work on the infection of tissue culture cell lines with the betacoronaviruses mouse hepatitis virus and severe acute respiratory syndrome coronavirus (SARS-CoV) showed that they generate double-membrane vesicles (DMVs) and convoluted membranes as part of a reticular membrane network. Here we describe a detailed study of the membrane rearrangements induced by the avian gammacoronavirus infectious bronchitis virus (IBV) in a mammalian cell line but also in primary avian cells and in epithelial cells of ex vivo tracheal organ cultures. In all cell types, structures novel to IBV infection were identified that we have termed zippered endoplasmic reticulum (ER) and spherules. Zippered ER lacked luminal space, suggesting zippering of ER cisternae, while spherules appeared as uniform invaginations of zippered ER. Electron tomography showed that IBV-induced spherules are tethered to the zippered ER and that there is a channel connecting the interior of the spherule with the cytoplasm, a feature thought to be necessary for sites of RNA synthesis but not seen previously for membrane rearrangements induced by coronaviruses. We also identified DMVs in IBV-infected cells that were observed as single individual DMVs or were connected to the ER via their outer membrane but not to the zippered ER. Interestingly, IBV-induced spherules strongly resemble confirmed sites of RNA synthesis for alphaviruses, nodaviruses, and bromoviruses, which may indicate similar strategies of IBV and these diverse viruses for the assembly of RNA replication complexes.
Importance: All positive-sense single-stranded RNA viruses induce rearranged cellular membranes, providing a platform for viral replication complex assembly and protecting viral RNA from cellular defenses. We have studied the membrane rearrangements induced by an important poultry pathogen, the gammacoronavirus infectious bronchitis virus (IBV). Previous work studying closely related betacoronaviruses identified double-membrane vesicles (DMVs) and convoluted membranes (CMs) derived from the endoplasmic reticulum (ER) in infected cells. However, the role of DMVs and CMs in viral RNA synthesis remains unclear because these sealed vesicles lack a means of delivering viral RNA to the cytoplasm. Here, we characterized structures novel to IBV infection: zippered ER and small vesicles tethered to the zippered ER termed spherules. Significantly, spherules contain a channel connecting their interior to the cytoplasm and strongly resemble confirmed sites of RNA synthesis for other positive-sense RNA viruses, making them ideal candidates for the site of IBV RNA synthesis.
Figures




Comment in
-
How the double spherules of infectious bronchitis virus impact our understanding of RNA virus replicative organelles.mBio. 2013 Dec 17;4(6):e00987-13. doi: 10.1128/mBio.00987-13. mBio. 2013. PMID: 24345746 Free PMC article.
-
Spherules and IBV.Bioengineered. 2014 Sep-Oct;5(5):288-92. doi: 10.4161/bioe.29323. Bioengineered. 2014. PMID: 25482229 Free PMC article.
References
-
- Cottam E, Pierini R, Roberts R, Wileman T. 2009. Origins of membrane vesicles generated during replication of positive-strand RNA viruses. Future Virol. 4:473–485
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
