Mesenchymal stem cells enhance autophagy and increase β-amyloid clearance in Alzheimer disease models
- PMID: 24149893
- PMCID: PMC4389879
- DOI: 10.4161/auto.26508
Mesenchymal stem cells enhance autophagy and increase β-amyloid clearance in Alzheimer disease models
Abstract
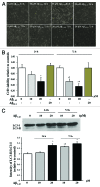
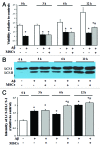
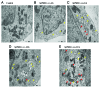
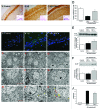
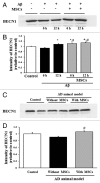
Current evidence suggests a central role for autophagy in Alzheimer disease (AD), and dysfunction in the autophagic system may lead to amyloid-β (Aβ) accumulation. Using in vitro and in vivo AD models, the present study investigated whether mesenchymal stem cells (MSCs) could enhance autophagy and thus exert a neuroprotective effect through modulation of Aβ clearance In Aβ-treated neuronal cells, MSCs increased cellular viability and enhanced LC3-II expression compared with cells treated with Aβ only. Immunofluorescence revealed that MSC coculture in Aβ-treated neuronal cells increased the number of LC3-II-positive autophagosomes that were colocalized with a lysosomal marker. Ultrastructural analysis revealed that most autophagic vacuoles (AVs) in Aβ-treated cells were not fused with lysosomes, whereas a large portion of autophagosomes were conjoined with lysosomes in MSCs cocultured with Aβ-treated neuronal cells. Furthermore, MSC coculture markedly increased Aβ immunoreactivity colocalized within lysosomes and decreased intracellular Aβ levels compared with Aβ-treated cells. In Aβ-treated animals, MSC administration significantly increased autophagosome induction, final maturation of late AVs, and fusion with lysosomes. Moreover, MSC administration significantly reduced the level of Aβ in the hippocampus, which was elevated in Aβ-treated mice, concomitant with increased survival of hippocampal neurons. Finally, MSC coculture upregulated BECN1/Beclin 1 expression in AD models. These results suggest that MSCs significantly enhance autolysosome formation and clearance of Aβ in AD models, which may lead to increased neuronal survival against Aβ toxicity. Modulation of the autophagy pathway to repair the damaged AD brain using MSCs would have a significant impact on future strategies for AD treatment.
Keywords: Alzheimer disease; BECN1; amyloid beta; autophagy; mesenchymal stem cell.
Figures



References
-
- Walsh DM, Selkoe DJ. . Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron 2004; 44:181 - 93; http://dx.doi.org/10.1016/j.neuron.2004.09.010; PMID: 15450169 - DOI - PubMed
-
- Sisodia SS, Price DL. . Role of the beta-amyloid protein in Alzheimer’s disease. FASEB J 1995; 9:366 - 70; PMID: 7896005 - PubMed
-
- Haass C, De Strooper B. . The presenilins in Alzheimer’s disease--proteolysis holds the key. Science 1999; 286:916 - 9; http://dx.doi.org/10.1126/science.286.5441.916; PMID: 10542139 - DOI - PubMed
-
- Yankner BA. . Mechanisms of neuronal degeneration in Alzheimer’s disease. Neuron 1996; 16:921 - 32; http://dx.doi.org/10.1016/S0896-6273(00)80115-4; PMID: 8630250 - DOI - PubMed
-
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ. . Autophagy fights disease through cellular self-digestion. Nature 2008; 451:1069 - 75; http://dx.doi.org/10.1038/nature06639; PMID: 18305538 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
