Multiple cytoskeletal pathways and PI3K signaling mediate CDC-42-induced neuronal protrusion in C. elegans
- PMID: 24149939
- PMCID: PMC4011816
- DOI: 10.4161/sgtp.26602
Multiple cytoskeletal pathways and PI3K signaling mediate CDC-42-induced neuronal protrusion in C. elegans
Abstract
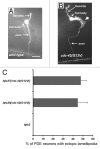
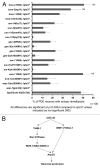
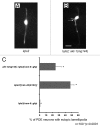
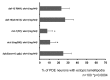
Rho GTPases are key regulators of cellular protrusion and are involved in many developmental events including axon guidance during nervous system development. Rho GTPase pathways display functional redundancy in developmental events, including axon guidance. Therefore, their roles can often be masked when using simple loss-of-function genetic approaches. As a complement to loss-of-function genetics, we constructed a constitutively activated CDC-42(G12V) expressed in C. elegans neurons. CDC-42(G12V) drove the formation of ectopic lamellipodial and filopodial protrusions in the PDE neurons, which resembled protrusions normally found on migrating growth cones of axons. We then used a candidate gene approach to identify molecules that mediate CDC-42(G12V)-induced ectopic protrusions by determining if loss of function of the genes could suppress CDC-42(G12V). Using this approach, we identified 3 cytoskeletal pathways previously implicated in axon guidance, the Arp2/3 complex, UNC-115/abLIM, and UNC-43/Ena. We also identified the Nck-interacting kinase MIG-15/NIK and p21-activated kinases (PAKs), also implicated in axon guidance. Finally, PI3K signaling was required, specifically the Rictor/mTORC2 branch but not the mTORC1 branch that has been implicated in other aspects of PI3K signaling including stress and aging. Our results indicate that multiple pathways can mediate CDC-42-induced neuronal protrusions that might be relevant to growth cone protrusions during axon pathfinding. Each of these pathways involves Rac GTPases, which might serve to integrate the pathways and coordinate the multiple CDC-42 pathways. These pathways might be relevant to developmental events such as axon pathfinding as well as disease states such as metastatic melanoma.
Keywords: AGE-1/PI3K; Arp2/3; CDC-42; MIG-15; UNC-115; axon pathfinding; mTORC2.
Figures





References
-
- Sulston J. J H. The nematode Caenorhabditis elegans. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 1988. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
