Transient activation of hedgehog pathway rescued irradiation-induced hyposalivation by preserving salivary stem/progenitor cells and parasympathetic innervation
- PMID: 24150232
- PMCID: PMC3951215
- DOI: 10.1158/1078-0432.CCR-13-1434
Transient activation of hedgehog pathway rescued irradiation-induced hyposalivation by preserving salivary stem/progenitor cells and parasympathetic innervation
Abstract
Purpose: To examine the effects and mechanisms of transient activation of the Hedgehog pathway on rescuing radiotherapy-induced hyposalivation in survivors of head and neck cancer.
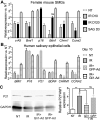
Experimental design: Mouse salivary glands and cultured human salivary epithelial cells were irradiated by a single 15-Gy dose. The Hedgehog pathway was transiently activated in mouse salivary glands, by briefly overexpressing the Sonic hedgehog (Shh) transgene or administrating smoothened agonist, and in human salivary epithelial cells, by infecting with adenovirus encoding Gli1. The activity of Hedgehog signaling was examined by the expression of the Ptch1-lacZ reporter and endogenous Hedgehog target genes. The salivary flow rate was measured following pilocarpine stimulation. Salivary stem/progenitor cells (SSPC), parasympathetic innervation, and expression of related genes were examined by flow cytometry, salisphere assay, immunohistochemistry, quantitative reverse transcription PCR, Western blotting, and ELISA.
Results: Irradiation does not activate Hedgehog signaling in mouse salivary glands. Transient Shh overexpression activated the Hedgehog pathway in ductal epithelia and, after irradiation, rescued salivary function in male mice, which is related with preservation of functional SSPCs and parasympathetic innervation. The preservation of SSPCs was likely mediated by the rescue of signaling activities of the Bmi1 and Chrm1-HB-EGF pathways. The preservation of parasympathetic innervation was associated with the rescue of the expression of neurotrophic factors such as Bdnf and Nrtn. The expression of genes related with maintenance of SSPCs and parasympathetic innervation in female salivary glands and cultured human salivary epithelial cells was similarly affected by irradiation and transient Hedgehog activation.
Conclusions: These findings suggest that transient activation of the Hedgehog pathway has the potential to restore salivary gland function after irradiation-induced dysfunction.
Figures





References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. - PubMed
-
- Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–41. - PubMed
-
- Jensen SB, Pedersen AM, Vissink A, Andersen E, Brown CG, Davies AN, et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: management strategies and economic impact. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2010;18:1061–79. - PubMed
-
- Konings AW, Coppes RP, Vissink A. On the mechanism of salivary gland radiosensitivity. Int J Radiat Oncol Biol Phys. 2005;62:1187–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

