Hypoxia-induced and A2A adenosine receptor-independent T-cell suppression is short lived and easily reversible
- PMID: 24150242
- PMCID: PMC3923523
- DOI: 10.1093/intimm/dxt045
Hypoxia-induced and A2A adenosine receptor-independent T-cell suppression is short lived and easily reversible
Abstract
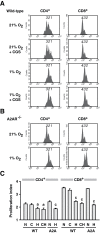
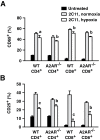
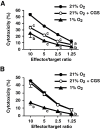
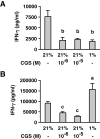
Tissue hypoxia plays a key role in establishing an immunosuppressive environment in vivo by, among other effects, increasing the level of extracellular adenosine, which then signals through A2A adenosine receptor (A2AR) to elicit its immunosuppressive effect. Although the important role of the adenosine--A2AR interaction in limiting inflammation has been established, the current study revisited this issue by asking whether hypoxia can also exert its T-cell inhibitory effects even without A2AR. A similar degree of hypoxia-triggered inhibition was observed in wild-type and A2AR-deficient T cells both in vitro and, after exposure of mice to a hypoxic atmosphere, in vivo. This A2AR-independent hypoxic T-cell suppression was qualitatively and mechanistically different from immunosuppression by A2AR stimulation. The A2AR-independent hypoxic immunosuppression strongly reduced T-cell proliferation, while IFN-γ-producing activity was more susceptible to the A2AR-dependent inhibition. In contrast to the sustained functional impairment after A2AR-mediated T-cell inhibition, the A2AR-independent inhibition under hypoxia was short lived, as evidenced by the quick recovery of IFN-γ-producing activity upon re-stimulation. These data support the view that T-cell inhibition by hypoxia can be mediated by multiple mechanisms and that both A2AR and key molecules in the A2AR-independent T-cell inhibition should be targeted to overcome the hypoxia-related immunosuppression in infected tissues and tumors.
Keywords: IFN-γ; immunosuppression; inflammation; oxygen; tumor microenvironment.
Figures


Similar articles
-
A2A Adenosine Receptor Gene Deletion or Synthetic A2A Antagonist Liberate Tumor-Reactive CD8+ T Cells from Tumor-Induced Immunosuppression.J Immunol. 2018 Jul 15;201(2):782-791. doi: 10.4049/jimmunol.1700850. Epub 2018 May 25. J Immunol. 2018. PMID: 29802128 Free PMC article.
-
Critical role of hypoxia and A2A adenosine receptors in liver tissue-protecting physiological anti-inflammatory pathway.Mol Med. 2008 Mar-Apr;14(3-4):116-23. doi: 10.2119/2007-00075.Chouker. Mol Med. 2008. PMID: 18163162 Free PMC article.
-
Extracellular purine metabolism and signaling of CD73-derived adenosine in murine Treg and Teff cells.Am J Physiol Cell Physiol. 2011 Aug;301(2):C530-9. doi: 10.1152/ajpcell.00385.2010. Epub 2011 May 18. Am J Physiol Cell Physiol. 2011. PMID: 21593451
-
Targeting Hypoxia-A2A Adenosinergic Immunosuppression of Antitumor T Cells During Cancer Immunotherapy.Front Immunol. 2020 Sep 29;11:570041. doi: 10.3389/fimmu.2020.570041. eCollection 2020. Front Immunol. 2020. PMID: 33117358 Free PMC article. Review.
-
Hypoxia-adenosinergic immunosuppression: tumor protection by T regulatory cells and cancerous tissue hypoxia.Clin Cancer Res. 2008 Oct 1;14(19):5947-52. doi: 10.1158/1078-0432.CCR-08-0229. Clin Cancer Res. 2008. PMID: 18829471 Review.
Cited by
-
Novel approaches to enhance the specificity and safety of engineered T cells.Cancer J. 2014 Mar-Apr;20(2):160-5. doi: 10.1097/PPO.0000000000000040. Cancer J. 2014. PMID: 24667964 Free PMC article.
-
Metabolic programming and immune suppression in the tumor microenvironment.Cancer Cell. 2023 Mar 13;41(3):421-433. doi: 10.1016/j.ccell.2023.01.009. Epub 2023 Feb 16. Cancer Cell. 2023. PMID: 36801000 Free PMC article. Review.
-
Myeloid cells in the tumor microenvironment: Role of adenosine.Oncoimmunology. 2015 Dec 3;5(3):e1108515. doi: 10.1080/2162402X.2015.1108515. eCollection 2016 Mar. Oncoimmunology. 2015. PMID: 27141365 Free PMC article. Review.
-
Adenosine-mediated immunosuppression in patients with squamous cell carcinoma of the head and neck.HNO. 2016 May;64(5):303-9. doi: 10.1007/s00106-016-0137-7. HNO. 2016. PMID: 27052632 Review. English.
-
Germinal Center Hypoxia Potentiates Immunoglobulin Class Switch Recombination.J Immunol. 2016 Nov 15;197(10):4014-4020. doi: 10.4049/jimmunol.1601401. Epub 2016 Oct 19. J Immunol. 2016. PMID: 27798169 Free PMC article.
References
-
- Lawrence T., Willoughby D. A., Gilroy D. W. 2002. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat. Rev. Immunol. 2:787. - PubMed
-
- Mellor A. L., Munn D. H. 2008. Creating immune privilege: active local suppression that benefits friends, but protects foes. Nat. Rev. Immunol. 8:74. - PubMed
-
- Sitkovsky M. V., Lukashev D., Apasov S., et al. 2004. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu. Rev. Immunol. 22:657. - PubMed
-
- Ohta A., Ohta A., Madasu M., et al. 2009. A2A adenosine receptor may allow expansion of T cells lacking effector functions in extracellular adenosine-rich microenvironments. J. Immunol. 183:5487. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

