Sequential induction of auxin efflux and influx carriers regulates lateral root emergence
- PMID: 24150423
- PMCID: PMC3817398
- DOI: 10.1038/msb.2013.43
Sequential induction of auxin efflux and influx carriers regulates lateral root emergence
Abstract
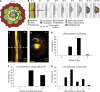
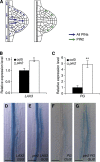
In Arabidopsis, lateral roots originate from pericycle cells deep within the primary root. New lateral root primordia (LRP) have to emerge through several overlaying tissues. Here, we report that auxin produced in new LRP is transported towards the outer tissues where it triggers cell separation by inducing both the auxin influx carrier LAX3 and cell-wall enzymes. LAX3 is expressed in just two cell files overlaying new LRP. To understand how this striking pattern of LAX3 expression is regulated, we developed a mathematical model that captures the network regulating its expression and auxin transport within realistic three-dimensional cell and tissue geometries. Our model revealed that, for the LAX3 spatial expression to be robust to natural variations in root tissue geometry, an efflux carrier is required--later identified to be PIN3. To prevent LAX3 from being transiently expressed in multiple cell files, PIN3 and LAX3 must be induced consecutively, which we later demonstrated to be the case. Our study exemplifies how mathematical models can be used to direct experiments to elucidate complex developmental processes.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





Comment in
-
Root systems analysis branches out.Mol Syst Biol. 2013 Oct 22;9:698. doi: 10.1038/msb.2013.51. Mol Syst Biol. 2013. PMID: 24150422 Free PMC article. No abstract available.
Similar articles
-
Lateral root emergence in Arabidopsis is dependent on transcription factor LBD29 regulation of auxin influx carrier LAX3.Development. 2016 Sep 15;143(18):3340-9. doi: 10.1242/dev.136283. Epub 2016 Aug 30. Development. 2016. PMID: 27578783
-
The novel cyst nematode effector protein 19C07 interacts with the Arabidopsis auxin influx transporter LAX3 to control feeding site development.Plant Physiol. 2011 Feb;155(2):866-80. doi: 10.1104/pp.110.167197. Epub 2010 Dec 14. Plant Physiol. 2011. PMID: 21156858 Free PMC article.
-
Arabidopsis SHR and SCR transcription factors and AUX1 auxin influx carrier control the switch between adventitious rooting and xylogenesis in planta and in in vitro cultured thin cell layers.Ann Bot. 2015 Mar;115(4):617-28. doi: 10.1093/aob/mcu258. Epub 2015 Jan 23. Ann Bot. 2015. PMID: 25617411 Free PMC article.
-
Spatiotemporal asymmetric auxin distribution: a means to coordinate plant development.Cell Mol Life Sci. 2006 Dec;63(23):2738-54. doi: 10.1007/s00018-006-6116-5. Cell Mol Life Sci. 2006. PMID: 17013565 Free PMC article. Review.
-
Deciphering Auxin-Ethylene Crosstalk at a Systems Level.Int J Mol Sci. 2018 Dec 14;19(12):4060. doi: 10.3390/ijms19124060. Int J Mol Sci. 2018. PMID: 30558241 Free PMC article. Review.
Cited by
-
Interplay of Auxin and Cytokinin in Lateral Root Development.Int J Mol Sci. 2019 Jan 23;20(3):486. doi: 10.3390/ijms20030486. Int J Mol Sci. 2019. PMID: 30678102 Free PMC article. Review.
-
Intracellularly Localized PIN-FORMED8 Promotes Lateral Root Emergence in Arabidopsis.Front Plant Sci. 2020 Jan 31;10:1808. doi: 10.3389/fpls.2019.01808. eCollection 2019. Front Plant Sci. 2020. PMID: 32082353 Free PMC article.
-
Far-Red Light Detection in the Shoot Regulates Lateral Root Development through the HY5 Transcription Factor.Plant Cell. 2018 Jan;30(1):101-116. doi: 10.1105/tpc.17.00771. Epub 2018 Jan 9. Plant Cell. 2018. PMID: 29321188 Free PMC article.
-
A tetraspanin gene regulating auxin response and affecting orchid perianth size and various plant developmental processes.Plant Direct. 2019 Aug 5;3(8):e00157. doi: 10.1002/pld3.157. eCollection 2019 Aug. Plant Direct. 2019. PMID: 31406958 Free PMC article.
-
Cloning and characterization of auxin efflux carrier genes EcPIN1a and EcPIN1b from finger millet Eleusine coracana L.3 Biotech. 2017 May;7(1):51. doi: 10.1007/s13205-017-0689-6. Epub 2017 Apr 25. 3 Biotech. 2017. PMID: 28444595 Free PMC article.
References
-
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 - PubMed
-
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B (2005) The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433: 39–44 - PubMed
-
- Frey PJF (2001) Medit 3.0 interactive mesh visualization software http://www.ann.jussieu.fr/~frey/software.html
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

