Changes in neural circuitry regulating response-reversal learning and Arc-mediated consolidation of learning in rats with methamphetamine-induced partial monoamine loss
- PMID: 24150570
- PMCID: PMC3924530
- DOI: 10.1038/npp.2013.296
Changes in neural circuitry regulating response-reversal learning and Arc-mediated consolidation of learning in rats with methamphetamine-induced partial monoamine loss
Abstract
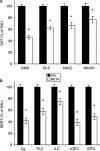
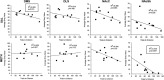
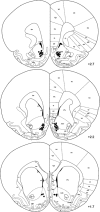
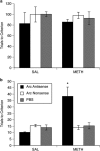
Methamphetamine (METH)-induced neurotoxicity results in long-lasting depletions of monoamines and changes in basal ganglia function. We previously reported that rats with METH-induced neurotoxicity no longer engage dorsomedial striatum during a response-reversal learning task, as their performance is insensitive to acute disruption of dorsomedial striatal function by local infusion of an N-methyl-D-aspartate receptor antagonist or an antisense oligonucleotide against the activity-regulated cytoskeleton-associated (Arc) gene. However, METH-pretreated rats perform the task as well as controls. Therefore, we hypothesized that the neural circuitry involved in the learning had changed in METH-pretreated rats. To test this hypothesis, rats were pretreated with a neurotoxic regimen of METH or with saline. After 3-5 weeks, rats were trained on the reversal-learning task and in situ hybridization for Arc was performed. A significant correlation between Arc expression and performance on the task was found in nucleus accumbens shell of METH-, but not saline-, pretreated rats. Consistent with the idea that the correlation between Arc expression in a brain region and behavioral performance implicates that brain region in the learning, infusion of an antisense oligonucleotide against Arc into the shell impaired consolidation of reversal learning in METH-, but not saline-, pretreated rats. These findings provide novel evidence suggesting that METH-induced neurotoxicity leads to a shift from dorsal to ventral striatal involvement in the reversal-learning task. Such reorganization of neural circuitry underlying learning and memory processes may contribute to impaired cognitive function in individuals with METH-induced neurotoxicity or others with striatal dopamine loss, such as patients with Parkinson's disease.
Figures
Similar articles
-
Altered learning and Arc-regulated consolidation of learning in striatum by methamphetamine-induced neurotoxicity.Neuropsychopharmacology. 2012 Mar;37(4):885-95. doi: 10.1038/npp.2011.265. Epub 2011 Nov 9. Neuropsychopharmacology. 2012. PMID: 22071872 Free PMC article.
-
Effect of methamphetamine neurotoxicity on learning-induced Arc mRNA expression in identified striatal efferent neurons.Neurotox Res. 2008 Dec;14(4):307-15. doi: 10.1007/BF03033855. Neurotox Res. 2008. PMID: 19073434 Free PMC article.
-
Disruption of subcellular Arc/Arg 3.1 mRNA expression in striatal efferent neurons following partial monoamine loss induced by methamphetamine.J Neurochem. 2012 Dec;123(5):845-55. doi: 10.1111/jnc.12017. Epub 2012 Oct 10. J Neurochem. 2012. PMID: 22978492 Free PMC article.
-
Current research on methamphetamine-induced neurotoxicity: animal models of monoamine disruption.J Pharmacol Sci. 2003 Jul;92(3):178-95. doi: 10.1254/jphs.92.178. J Pharmacol Sci. 2003. PMID: 12890883 Review.
-
Methamphetamine addiction: involvement of CREB and neuroinflammatory signaling pathways.Psychopharmacology (Berl). 2016 May;233(10):1945-62. doi: 10.1007/s00213-016-4235-8. Epub 2016 Feb 12. Psychopharmacology (Berl). 2016. PMID: 26873080 Free PMC article. Review.
Cited by
-
Immediate Early Genes, Memory and Psychiatric Disorders: Focus on c-Fos, Egr1 and Arc.Front Behav Neurosci. 2018 Apr 25;12:79. doi: 10.3389/fnbeh.2018.00079. eCollection 2018. Front Behav Neurosci. 2018. PMID: 29755331 Free PMC article. Review.
-
Activity-Dependent Arc Expression and Homeostatic Synaptic Plasticity Are Altered in Neurons from a Mouse Model of Angelman Syndrome.Front Mol Neurosci. 2017 Jul 28;10:234. doi: 10.3389/fnmol.2017.00234. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28804447 Free PMC article.
-
Nanowired Delivery of Curcumin Attenuates Methamphetamine Neurotoxicity and Elevates Levels of Dopamine and Brain-Derived Neurotrophic Factor.Adv Neurobiol. 2023;32:385-416. doi: 10.1007/978-3-031-32997-5_10. Adv Neurobiol. 2023. PMID: 37480467
-
Exercise induction at expression immediate early gene (c-Fos, ARC, EGR-1) in the hippocampus: a systematic review.Dement Neuropsychol. 2024 Apr 15;18:e20230015. doi: 10.1590/1980-5764-DN-2023-0015. eCollection 2024. Dement Neuropsychol. 2024. PMID: 38628561 Free PMC article. Review.
References
-
- Beauchamp MH, Dagher A, Panisset M, Doyon J. Neural substrates of cognitive skill learning in Parkinson's disease. Brain Cogn. 2008;68:134–143. - PubMed
-
- Boja JW, Mitchell WM, Patel A, Kopajtic TA, Carroll FI, Lewin AH, et al. High-affinity binding of [125I]RTI-55 to dopamine and serotonin transporters in rat brain. Synapse. 1992;12:27–36. - PubMed
-
- Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30:211–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

