Comet assay in reconstructed 3D human epidermal skin models--investigation of intra- and inter-laboratory reproducibility with coded chemicals
- PMID: 24150594
- PMCID: PMC4023299
- DOI: 10.1093/mutage/get051
Comet assay in reconstructed 3D human epidermal skin models--investigation of intra- and inter-laboratory reproducibility with coded chemicals
Abstract
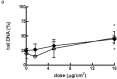
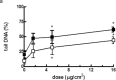
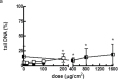
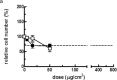
Reconstructed 3D human epidermal skin models are being used increasingly for safety testing of chemicals. Based on EpiDerm™ tissues, an assay was developed in which the tissues were topically exposed to test chemicals for 3h followed by cell isolation and assessment of DNA damage using the comet assay. Inter-laboratory reproducibility of the 3D skin comet assay was initially demonstrated using two model genotoxic carcinogens, methyl methane sulfonate (MMS) and 4-nitroquinoline-n-oxide, and the results showed good concordance among three different laboratories and with in vivo data. In Phase 2 of the project, intra- and inter-laboratory reproducibility was investigated with five coded compounds with different genotoxicity liability tested at three different laboratories. For the genotoxic carcinogens MMS and N-ethyl-N-nitrosourea, all laboratories reported a dose-related and statistically significant increase (P < 0.05) in DNA damage in every experiment. For the genotoxic carcinogen, 2,4-diaminotoluene, the overall result from all laboratories showed a smaller, but significant genotoxic response (P < 0.05). For cyclohexanone (CHN) (non-genotoxic in vitro and in vivo, and non-carcinogenic), an increase compared to the solvent control acetone was observed only in one laboratory. However, the response was not dose related and CHN was judged negative overall, as was p-nitrophenol (p-NP) (genotoxic in vitro but not in vivo and non-carcinogenic), which was the only compound showing clear cytotoxic effects. For p-NP, significant DNA damage generally occurred only at doses that were substantially cytotoxic (>30% cell loss), and the overall response was comparable in all laboratories despite some differences in doses tested. The results of the collaborative study for the coded compounds were generally reproducible among the laboratories involved and intra-laboratory reproducibility was also good. These data indicate that the comet assay in EpiDerm™ skin models is a promising model for the safety assessment of compounds with a dermal route of exposure.
Figures
























Similar articles
-
Validation of the 3D Skin Comet assay using full thickness skin models: Transferability and reproducibility.Mutat Res Genet Toxicol Environ Mutagen. 2018 Mar;827:27-41. doi: 10.1016/j.mrgentox.2018.01.003. Epub 2018 Jan 10. Mutat Res Genet Toxicol Environ Mutagen. 2018. PMID: 29502735
-
Intralaboratory and interlaboratory evaluation of the EpiDerm 3D human reconstructed skin micronucleus (RSMN) assay.Mutat Res. 2009 Mar 17;673(2):100-8. doi: 10.1016/j.mrgentox.2008.12.003. Epub 2009 Jan 9. Mutat Res. 2009. PMID: 19167513
-
Investigation of comet assays under conditions mimicking ocular instillation administration in a three-dimensional reconstructed human corneal epithelial model.Cutan Ocul Toxicol. 2019 Dec;38(4):375-383. doi: 10.1080/15569527.2019.1634580. Epub 2019 Jul 8. Cutan Ocul Toxicol. 2019. PMID: 31223032
-
The comet assay with multiple mouse organs: comparison of comet assay results and carcinogenicity with 208 chemicals selected from the IARC monographs and U.S. NTP Carcinogenicity Database.Crit Rev Toxicol. 2000 Nov;30(6):629-799. doi: 10.1080/10408440008951123. Crit Rev Toxicol. 2000. PMID: 11145306 Review.
-
Influence of mus201 and mus308 mutations of Drosophila melanogaster on the genotoxicity of model chemicals in somatic cells in vivo measured with the comet assay.Mutat Res. 2002 Jun 19;503(1-2):11-9. doi: 10.1016/s0027-5107(02)00070-2. Mutat Res. 2002. PMID: 12052499 Review.
Cited by
-
Three dimensional and microphysiological bone marrow models detect in vivo positive compounds.Sci Rep. 2021 Nov 9;11(1):21959. doi: 10.1038/s41598-021-01400-5. Sci Rep. 2021. PMID: 34754012 Free PMC article.
-
Extracellular signal-regulated kinases 1/2 and Akt contribute to triclosan-stimulated proliferation of JB6 Cl 41-5a cells.Arch Toxicol. 2015 Aug;89(8):1297-311. doi: 10.1007/s00204-014-1308-5. Epub 2014 Jul 18. Arch Toxicol. 2015. PMID: 25033989 Free PMC article.
-
State-of-the-art of 3D cultures (organs-on-a-chip) in safety testing and pathophysiology.ALTEX. 2014;31(4):441-77. doi: 10.14573/altex.1406111. Epub 2014 Jul 14. ALTEX. 2014. PMID: 25027500 Free PMC article. Review.
-
Measuring DNA modifications with the comet assay: a compendium of protocols.Nat Protoc. 2023 Mar;18(3):929-989. doi: 10.1038/s41596-022-00754-y. Epub 2023 Jan 27. Nat Protoc. 2023. PMID: 36707722 Free PMC article. Review.
-
Genetic toxicity testing using human in vitro organotypic airway cultures: Assessing DNA damage with the CometChip and mutagenesis by Duplex Sequencing.Environ Mol Mutagen. 2021 Jun;62(5):306-318. doi: 10.1002/em.22444. Epub 2021 Jun 14. Environ Mol Mutagen. 2021. PMID: 34050964 Free PMC article.
References
-
- Kirkland D., Aardema M., Henderson L., Müller L. (2005). Evaluation of the ability of a battery of three in vitro genotoxicity tests to discriminate rodent carcinogens and non-carcinogens I. Sensitivity, specificity and relative predictivity. Mutat. Res., 584, 1–256 - PubMed
-
- Kirkland D., Aardema M., Müller L., Makoto H. (2006). Evaluation of the ability of a battery of three in vitro genotoxicity tests to discriminate rodent carcinogens and non-carcinogens II. Further analysis of mammalian cell results, relative predictivity and tumour profiles. Mutat. Res., 608, 29–42 - PubMed
-
- Kirkland D., Pfuhler S., Tweats D., et al. (2007). How to reduce false positive results when undertaking in vitro genotoxicity testing and thus avoid unnecessary follow-up animal tests: report of an ECVAM Workshop. Mutat. Res., 628, 31–55 - PubMed
-
- Matthews E. J., Kruhlak N. L., Cimino M. C., Benz R. D., Contrera J. F. (2006). An analysis of genetic toxicity, reproductive and developmental toxicity, and carcinogenicity data: I. Identification of carcinogens using surrogate endpoints. Regul. Toxicol. Pharmacol., 44, 83–96 - PubMed
-
- EC 1907/2006. (2006). Regulation (EC) No 1907/2006 of the European Parliament and the Council of 18 December 2006, concerning the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH), establishing a European Chemical Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/03 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EC, 93/67/EC, 93/105/EC and 2000/21/EC. Official J. Eur. Union, 49, 1–849
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

