Single dose oral celecoxib for acute postoperative pain in adults
- PMID: 24150982
- PMCID: PMC6463992
- DOI: 10.1002/14651858.CD004233.pub4
Single dose oral celecoxib for acute postoperative pain in adults
Abstract
Background: This is an update of a review first published in The Cochrane Library in Issue 4, 2008, and updated in Issue 3, 2012. Celecoxib is a selective cyclo-oxygenase-2 (COX-2) inhibitor usually prescribed for the relief of chronic pain in osteoarthritis and rheumatoid arthritis. Celecoxib is believed to be associated with fewer upper gastrointestinal adverse effects than conventional non-steroidal anti-inflammatory drugs (NSAIDs). Its effectiveness in acute pain was demonstrated in the earlier reviews.
Objectives: To assess analgesic efficacy and adverse effects of a single oral dose of celecoxib for moderate to severe postoperative pain in adults.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, the Oxford Pain Database, and ClinicalTrials.gov. The most recent search was to 31 May 2013.
Selection criteria: We included randomised, double-blind, placebo-controlled trials (RCTs) of adults prescribed any dose of oral celecoxib or placebo for acute postoperative pain.
Data collection and analysis: Two review authors assessed studies for quality and extracted data. We converted summed pain relief (TOTPAR) or pain intensity difference (SPID) into dichotomous information, yielding the number of participants with at least 50% pain relief over four to six hours. We used this to calculate the relative benefit (RB) and number needed to treat to benefit (NNT), for one patient to achieve at least 50% of maximum pain relief with celecoxib who would not have done so with placebo. We used information on use of rescue medication to calculate the proportion of participants requiring rescue medication and the weighted mean of the median time to use.
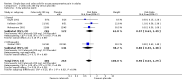
Main results: Ten studies (1785 participants) met the inclusion criteria. The two new studies in this update had been identified in the earlier update, but data were not available. There remain three potentially relevant unpublished studies for which data are not available at this time.The NNT for celecoxib 200 mg and 400 mg compared with placebo for at least 50% of maximum pain relief over four to six hours was 4.2 (95% confidence interval (CI) 3.4 to 5.6) and 2.6 (95% CI 2.3 to 3.0) respectively. The median time to use of rescue medication was 6.6 hours with celecoxib 200 mg, 8.4 hours with celecoxib 400 mg, and 2.3 hours with placebo. The proportion of participants requiring rescue medication over 24 hours was 74% with celecoxib 200 mg, 63% for celecoxib 400 mg, and 91% for placebo. The NNT to prevent one patient using rescue medication was 4.8 (95% CI 3.5 to 7.7) and 3.5 (95% CI 2.9 to 4.6) for celecoxib 200 mg and 400 mg respectively. Adverse events were generally mild to moderate in severity, and were experienced by a similar proportion of participants in the celecoxib and placebo groups. One serious adverse event that was probably related to celecoxib was reported.
Authors' conclusions: Single-dose oral celecoxib is an effective analgesic for postoperative pain relief. Indirect comparison suggests that the 400 mg dose has similar efficacy to ibuprofen 400 mg.
Conflict of interest statement
SD and TW have no interests to declare. RAM has consulted for various pharmaceutical companies and received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions. SD and RAM have received research support from charities, government, and industry sources at various times; no such support was received for this work.
Figures

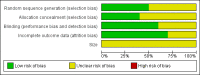
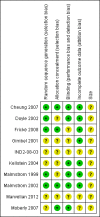

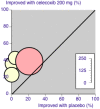

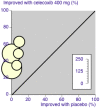





Update of
-
Single dose oral celecoxib for acute postoperative pain in adults.Cochrane Database Syst Rev. 2012 Mar 14;3(3):CD004233. doi: 10.1002/14651858.CD004233.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2013 Oct 22;(10):CD004233. doi: 10.1002/14651858.CD004233.pub4. PMID: 22419293 Free PMC article. Updated.
References
References to studies included in this review
Cheung 2007 {published data only}
-
- Cheung R, Krishnaswami S, Kowalski K. Analgesic efficacy of celecoxib in postoperative oral surgery pain: a single‐dose, two‐center, randomized, double‐blind, active‐ and placebo‐controlled study. Clinical Therapeutics 2007;29 Suppl:2498‐510. - PubMed
Doyle 2002 {published data only}
-
- Doyle G, Jayawardena S, Ashraf E, Cooper SA. Efficacy and tolerability of nonprescription ibuprofen versus celecoxib for dental pain. Journal of Clinical Pharmacology 2002;42(8):912‐9. - PubMed
Fricke 2008 {published data only}
-
- Fricke J, Davis N, Yu V, Krammer G. Lumiracoxib 400 mg compared with celecoxib 400 mg and placebo for treating pain following dental surgery: a randomized, controlled trial. The Journal of Pain 2008;9(1):20‐7. - PubMed
Gimbel 2001 {published data only}
-
- Gimbel JS, Brugger A, Zhao W, Verburg KM, Geis GS. Efficacy and tolerability of celecoxib versus hydrocodone/acetaminophen in the treatment of pain after ambulatory orthopedic surgery in adults. Clinical Therapeutics 2001;23(2):228‐41. - PubMed
IND2‐08‐03 {unpublished data only}
-
- A phase 2, randomized, double‐blind, single‐dose, parallel‐group, active‐ and placebo‐controlled study of indomethacin test capsules for the treatment of pain after surgical removal of impacted third molars. http://www.clinicaltrials.gov/ 2013 (Accessed 31 May 2013). [CTG: NCT00964431]
Kellstein 2004 {published data only}
-
- Kellstein D, Ott D, Jayawardene S, Fricke J. Analgesic efficacy of a single dose of lumiracoxib compared with rofecoxib, celecoxib and placebo in the treatment of post‐operative dental pain. International Journal of Clinical Practice 2004;58(3):244‐50. - PubMed
Malmstrom 1999 {published data only}
-
- Malmstrom K, Daniels S, Kotey P, Seidenburg BC, Desjardins PJ. Comparison of rofecoxib and celecoxib, two cyclooxygenase‐2 inhibitors, in postoperative dental pain: a randomised, placebo‐ and active‐comparator‐controlled clinical trial. Clinical Therapeutics 1999;21(10):1653‐63. - PubMed
Malmstrom 2002 {published data only}
-
- Malmstrom K, Fricke JR, Kotey P, Kress B, Morrison B. A comparison of rofecoxib versus celecoxib in treating pain after dental surgery: a single‐center, randomized, double‐blind, placebo‐ and active‐comparator‐controlled, parallel‐group, single‐dose study using the dental impaction pain model. Clinical Therapeutics 2002;24(10):1549‐60. - PubMed
Manvelian 2012 {published data only}
Moberly 2007 {published data only}
-
- Moberly JB, Xu J, Desjardins PJ, Daniels SE, Bandy DP, Lawson JE, et al. A randomized, double‐blind, celecoxib‐ and placebo‐controlled study of the effectiveness of CS‐706 in acute postoperative dental pain. Clinical Therapeutics 2007;29(3):399‐412. - PubMed
References to studies excluded from this review
Saito 2012 {published data only}
-
- Saito K, Kaneko A, Machii K, Ohta H, Ohkura M, Suzuki M. Efficacy and safety of additional 200‐mg dose of celecoxib in adult patients with postoperative pain following extraction of impacted third mandibular molar: a multicenter, randomized, double‐blind, placebo‐controlled, phase II study in Japan. Clinical Therapeutics 2012;34(2):314‐28. [DOI: 10.1016/j.clinthera.2012.01.004] - DOI - PubMed
Salo 2003 {published data only}
-
- Salo DF, Lavery R, Varma V, Goldberg J, Shapiro T, Kenwood A. A randomized, clinical trial comparing oral celecoxib 200 mg, celecoxib 400 mg, and ibuprofen 600 mg for acute pain. Academic Emergency Medicine 2003;10:22‐30. - PubMed
White 2007 {published data only}
-
- White PF, Sacan O, Tufanogullari B, Eng M, Nuangchamnong N, Ogunnaike B. Effect of short‐term postoperative celecoxib administration on patient outcome after outpatient laparoscopic surgery. Canadian Journal of Anaesthesia 2007;54(5):342‐8. - PubMed
References to studies awaiting assessment
177‐CL‐102 {unpublished data only}
-
- An etodolac‐ and placebo‐controlled, multicenter, double‐blind, group comparison study to verify the efficacy of YM177 (celecoxib) in postoperative pain patients. [CTG: NCT01118572]
ARRY‐797‐222 {unpublished data only}
-
- A randomized, double‐blind, placebo‐ and active‐controlled, parallel‐group analgesic efficacy trial of oral ARRY‐371797 in subjects undergoing third molar extraction. [CTG: NCT00663767]
Shirota 2001 {published data only}
-
- Shirota T, Ohno K‐S, Michii K‐I, Kamijo R, Nagumo M, Sato H, et al. A study of the dose‐response of YM177 for treatment of postsurgical dental pain. Oral Therapeutics and Pharmacology 2001;20(3):154‐72.
Additional references
Barden 2004
Clarke 2012
Collins 1997
-
- Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres?. Pain 1997;72:95‐7. - PubMed
Collins 2001
-
- Collins SL, Edwards J, Moore RA, Smith LA, McQuay HJ. Seeking a simple measure of analgesia for mega‐trials: is a single global assessment good enough?. Pain 2001;91:189‐94. - PubMed
Cook 1995
Cooper 1991
-
- Cooper SA. Single‐dose analgesic studies: the upside and downside of assay sensitivity. In: Max MB, Portenoy RK, Laska EM editor(s). The Design of Analgesic ClinicalTrials. Advances in Pain Research and Therapy. Vol. 18, New York: Raven Press, 1991:117‐24.
Derry 2011
Derry 2012
Derry C 2009a
Derry C 2009b
Derry P 2009
Edwards 1999a
-
- Edwards JE, Oldman AD, Smith LA, Carroll D, Wiffen PJ, McQuay HJ, et al. Oral aspirin in postoperative pain: a quantitative systematic review. Pain 1999;81:289‐97. - PubMed
Edwards 1999b
-
- Edwards JE, McQuay HJ, Moore RA, Collins SL. Reporting of adverse effects in clinical trials should be improved: lessons from acute postoperative pain. Journal of Pain and Symptom Management 1999;18(6):427‐37. - PubMed
Garner 2002
Grahame‐Smith 2002
-
- Grahame‐Smith DG, Aronson JK. Oxford Textbook of Clinical Pharmacology and Drug Therapy. 3rd Edition. Oxford: Oxford University Press, 2002.
Hawkey 1999
-
- Hawkey CJ. Cox‐2 inhibitors. Lancet 1999;353(9149):307‐14. - PubMed
Hawkey 2001
-
- Hawkey, CJ. Gastrointestinal safety of COX‐2 specific inhibitors. Gastroenterology Clinics of North America 2001;30(4):921‐36. - PubMed
Higgins 2011
-
- Altman DG, Antes G, Gøtzsche P, Higgins JPT, Jüni P, Lewis S, et al. Assessing risk of bias in included studies. In: Higgins JPT, Altman DG, Sterne JAC editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. www.cochrane‐handbook.org. The Cochrane Collaboration, 2011.
Jadad 1996a
-
- Jadad A, Carroll D, Moore RA, McQuay HJ. Developing a database of published reports of randomised clinical trials in pain research. Pain 1996;66:239‐46. - PubMed
Jadad 1996b
-
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. - PubMed
L'Abbé 1987
-
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107:224‐33. - PubMed
McQuay 2005
Moher 1999
-
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta‐analyses. Lancet 1999;27(354):1896‐900. - PubMed
Moore 1996
-
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics. Pain 1996;66(2‐3):229‐37. - PubMed
Moore 1997a
-
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: verification from independent data. Pain 1997;69(1‐2):127‐30. - PubMed
Moore 1997b
-
- Moore A, Moore O, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: use of pain intensity and visual analogue scales. Pain 1997;69(3):311‐5. - PubMed
Moore 1998
-
- Moore RA, Gavaghan D, Tramer M, Collins SL, McQuay HJ. Size is everything ‐ large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. - PubMed
Moore 2003
-
- Moore RA, Edwards J, Barden J, McQuay HJ. Bandolier's Little Book of Pain. Oxford: Oxford University Press, 2003.
Moore 2005a
-
- Moore RA, Edwards JE, McQuay HJ. Acute pain: individual patient meta‐analysis shows the impact of different ways of analysing and presenting results. Pain 2005;116(3):322‐31. - PubMed
Moore 2005b
-
- Moore RA, Derry S, Makinson GT, McQuay HJ. Tolerability and adverse events in clinical trials of celecoxib in osteoarthritis and rheumatoid arthritis: systematic review and meta‐analysis of information from company clinical trial reports. Arthritis Research and Therapy 2005;7(3):R644‐65. - PMC - PubMed
Moore 2006
-
- Moore A, McQuay H. Bandolier's Little Book of Making Sense of the Medical Evidence. Oxford: Oxford University Press, 2006.
Moore 2008
-
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24. [ISBN: 978‐0‐931092‐69‐5]
Moore 2011a
Moore 2011b
-
- Moore RA, Straube S, Paine J, Derry S, McQuay HJ. Minimum efficacy criteria for comparisons between treatments using individual patient meta‐analysis of acute pain trials: examples of etoricoxib, paracetamol, ibuprofen, and ibuprofen/paracetamol combinations after third molar extraction. Pain 2011;152(5):982‐9. [DOI: ] - PubMed
Morris 1995
Nuesch 2010
PACT 2010
-
- Prescription Cost Analysis England. The NHS Information Centre for Health and Social Care 2010. [ISBN: 978‐1‐84636‐542‐3]
Patrono 2009
-
- Patrono C, Baigent C. Low‐dose aspirin, coxibs, and other NSAIDS: a clinical mosaic emerges. Molecular Interventions 2009;9(1):31‐9. - PubMed
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Roy 2010
Ruff 2011
-
- Ruff CT, Morrow DA, Jarolim P, Ren F, Contant CF, Kaur A, et al. Evaluation of NT‐proBNP and high sensitivity C‐reactive protein for predicting cardiovascular risk in patients with arthritis taking longterm nonsteroidal antiinflammatory drugs. Journal of Rheumatology 2011;38(6):1071‐8. [DOI: 10.3899/jrheum.100880] - DOI - PubMed
Straube 2005
-
- Straube S, Derry S, McQuay HJ, Moore RA. Effect of preoperative Cox‐II‐selective NSAIDs (coxibs) on postoperative outcomes: a systematic review of randomized studies. Acta Anaesthesiologica Scandinavica 2005;49(5):601‐13. - PubMed
Toms 2008
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

