The R2R3-MYB transcription factors MYB14 and MYB15 regulate stilbene biosynthesis in Vitis vinifera
- PMID: 24151295
- PMCID: PMC3877794
- DOI: 10.1105/tpc.113.117127
The R2R3-MYB transcription factors MYB14 and MYB15 regulate stilbene biosynthesis in Vitis vinifera
Abstract
Plant stilbenes are phytoalexins that accumulate in a small number of plant species, including grapevine (Vitis vinifera), in response to biotic and abiotic stresses and have been implicated in many beneficial effects on human health. In particular, resveratrol, the basic unit of all other complex stilbenes, has received widespread attention because of its cardio-protective, anticarcinogenic, and antioxidant properties. Although stilbene synthases (STSs), the key enzymes responsible for resveratrol biosynthesis, have been isolated and characterized from several plant species, the transcriptional regulation underlying stilbene biosynthesis is unknown. Here, we report the identification and functional characterization of two R2R3-MYB-type transcription factors (TFs) from grapevine, which regulate the stilbene biosynthetic pathway. These TFs, designated MYB14 and MYB15, strongly coexpress with STS genes, both in leaf tissues under biotic and abiotic stress and in the skin and seed of healthy developing berries during maturation. In transient gene reporter assays, MYB14 and MYB15 were demonstrated to specifically activate the promoters of STS genes, and the ectopic expression of MYB15 in grapevine hairy roots resulted in increased STS expression and in the accumulation of glycosylated stilbenes in planta. These results demonstrate the involvement of MYB14 and MYB15 in the transcriptional regulation of stilbene biosynthesis in grapevine.
Figures


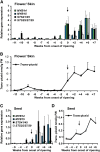


References
-
- Adrian M., Jeandet P., Bessis R., Joubert J.M. (1996). Induction of phytoalexin (resveratrol) synthesis in grapevine leaves treated with aluminum chloride (AlCl3). J. Agric. Food Chem. 44: 1979–1981
-
- Adrian M., Jeandet P., Veneau J., Weston L.A., Bessis R. (1997). Biological activity of resveratrol, a stilbenic compound from grapevines, against Botrytis cinerea, the causal agent for gray mold. J. Chem. Ecol. 23: 1689–1702
-
- Agarwal B., Baur J.A. (2011). Resveratrol and life extension. Ann. N. Y. Acad. Sci. 1215: 138–143 - PubMed
-
- Bais A.J., Murphy P.J., Dry I.B. (2000). The molecular regulation of stilbene phytoalexin biosynthesis in Vitis vinifera during grape berry development. Funct. Plant Biol. 27: 425–433
-
- Baur J.A., Sinclair D.A. (2006). Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 5: 493–506 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

