Ethanolamide oxylipins of linolenic acid can negatively regulate Arabidopsis seedling development
- PMID: 24151297
- PMCID: PMC3877782
- DOI: 10.1105/tpc.113.119024
Ethanolamide oxylipins of linolenic acid can negatively regulate Arabidopsis seedling development
Abstract
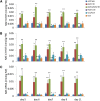
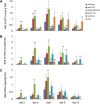
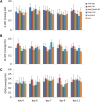
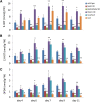
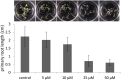
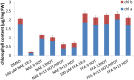
N-Acylethanolamines (NAEs) are fatty-acid derivatives with potent biological activities in a wide range of eukaryotic organisms. Polyunsaturated NAEs are among the most abundant NAE types in seeds of Arabidopsis thaliana, and they can be metabolized by either fatty acid amide hydrolase (FAAH) or by lipoxygenase (LOX) to low levels during seedling establishment. Here, we identify and quantify endogenous oxylipin metabolites of N-linolenoylethanolamine (NAE 18:3) in Arabidopsis seedlings and show that their levels were higher in faah knockout seedlings. Quantification of oxylipin metabolites in lox mutants demonstrated altered partitioning of NAE 18:3 into 9- or 13-LOX pathways, and this was especially exaggerated when exogenous NAE was added to seedlings. When maintained at micromolar concentrations, NAE 18:3 specifically induced cotyledon bleaching of light-grown seedlings within a restricted stage of development. Comprehensive oxylipin profiling together with genetic and pharmacological interference with LOX activity suggested that both 9-hydroxy and 13-hydroxy linolenoylethanolamides, but not corresponding free fatty-acid metabolites, contributed to the reversible disruption of thylakoid membranes in chloroplasts of seedling cotyledons. We suggest that NAE oxylipins of linolenic acid represent a newly identified, endogenous set of bioactive compounds that may act in opposition to progression of normal seedling development and must be depleted for successful establishment.
Figures









Comment in
-
Ethanolamide oxylipins: new players in seedling development.Plant Cell. 2013 Oct;25(10):3637. doi: 10.1105/tpc.113.251012. Epub 2013 Oct 22. Plant Cell. 2013. PMID: 24151296 Free PMC article. No abstract available.
References
-
- Bachur N.R., Masek K., Melmon K.L., Udenfriend S. (1965). Fatty acid amides of ethanolamine in mammalian tissues. J. Biol. Chem. 240: 1019–1024 - PubMed
-
- Blancaflor E.B., Hou G., Chapman K.D. (2003). Elevated levels of N-lauroylethanolamine, an endogenous constituent of desiccated seeds, disrupt normal root development in Arabidopsis thaliana seedlings. Planta 217: 206–217 - PubMed
-
- Brash A.R. (1999). Lipoxygenases: Occurrence, functions, catalysis, and acquisition of substrate. J. Biol. Chem. 274: 23679–23682 - PubMed
-
- Browse J. (2009). Jasmonate passes muster: A receptor and targets for the defense hormone. Annu. Rev. Plant Biol. 60: 183–205 - PubMed
-
- Chapman K.D. (2004). Occurrence, metabolism, and prospective functions of N-acylethanolamines in plants. Prog. Lipid Res. 43: 302–327 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

