Epstein-Barr virus-driven lymphomagenesis in the context of human immunodeficiency virus type 1 infection
- PMID: 24151490
- PMCID: PMC3799006
- DOI: 10.3389/fmicb.2013.00311
Epstein-Barr virus-driven lymphomagenesis in the context of human immunodeficiency virus type 1 infection
Abstract
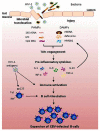
Epstein-Barr virus (EBV) is a ubiquitous human γ-herpes virus which establishes a life-long asymptomatic infection in immunocompetent hosts. In human immunodeficiency virus type 1 (HIV-1) infected patients, the impaired immunosurveillance against EBV may favor the development of EBV-related diseases, ranging from lymphoproliferative disorders to B cell non-Hodgkin's lymphomas (NHL). Antiretroviral therapy (ART) has significantly modified the natural course of HIV-1 infection, resulting in decreased HIV-1 plasmaviremia, increased CD4 lymphocytes, and decreased opportunistic infections, indicating a restoration of immune functions. However, the impact of ART appears to be less favorable on EBV-related malignancies than on other AIDS-defining tumors, such as Kaposi's sarcoma, and NHL remains the most common cancer during the ART era. EBV-driven tumors are associated with selective expression of latent oncogenic proteins, but uncontrolled lytic cycle with virus replication and/or reactivation may favor cell transformation, at least in the early phases. Several host's factors may promote EBV reactivation and replication; besides immunodepression, inflammation/chronic immune stimulation may play an important role. Microbial pathogen-associated molecular patterns and endogenous damage-associated molecular patterns, through Toll-like receptors, activate the immune system and may promote EBV reactivation and/or polyclonal expansion of EBV-infected cells. A body of evidence suggests that chronic immune stimulation is a hallmark of HIV-1 pathogenesis and may persist even in ART-treated patients. This review focuses on lymphomagenesis driven by EBV both in the context of the natural history of HIV-1 infection and in ART-treated patients. Understanding the mechanisms involved in the expansion of EBV-infected cells is a premise for the identification of prognostic markers of EBV-associated malignancies.
Keywords: B cell activation; EBV; EBV lytic reactivation; EBV-related malignancies; HIV-1; antiretroviral therapy; chronic immune activation.
Figures
Similar articles
-
Direct Epstein-Barr virus (EBV) typing on peripheral blood mononuclear cells: no association between EBV type 2 infection or superinfection and the development of acquired immunodeficiency syndrome-related non-Hodgkin's lymphoma.Blood. 1999 Jun 1;93(11):3949-55. Blood. 1999. PMID: 10339504
-
Epstein-Barr virus latent and replicative gene expression in post-transplant lymphoproliferative disorders and AIDS-related non-Hodgkin's lymphomas. French Study Group of Pathology for HIV-associated Tumors.Ann Oncol. 1994;5 Suppl 1:113-6. doi: 10.1093/annonc/5.suppl_1.s113. Ann Oncol. 1994. PMID: 8172807
-
Expression of the simian Epstein-Barr virus-encoded latent membrane protein-1 in malignant lymphomas of SIV-infected rhesus macaques.J Med Virol. 2001 Sep;65(1):114-20. J Med Virol. 2001. PMID: 11505452
-
Post-transplant lymphoproliferative disorders: from epidemiology to pathogenesis-driven treatment.Cancer Lett. 2015 Dec 1;369(1):37-44. doi: 10.1016/j.canlet.2015.08.007. Epub 2015 Aug 13. Cancer Lett. 2015. PMID: 26279520 Review.
-
Immunology of EBV-Related Lymphoproliferative Disease in HIV-Positive Individuals.Front Oncol. 2020 Sep 30;10:1723. doi: 10.3389/fonc.2020.01723. eCollection 2020. Front Oncol. 2020. PMID: 33102204 Free PMC article. Review.
Cited by
-
Felis Catus Gammaherpesvirus 1 DNAemia in Whole Blood from Therapeutically Immunosuppressed or Retrovirus-Infected Cats.Vet Sci. 2017 Mar 14;4(1):16. doi: 10.3390/vetsci4010016. Vet Sci. 2017. PMID: 29056675 Free PMC article.
-
Cytokine Storm Syndromes Associated with Epstein-Barr Virus.Adv Exp Med Biol. 2024;1448:227-248. doi: 10.1007/978-3-031-59815-9_16. Adv Exp Med Biol. 2024. PMID: 39117818 Review.
-
Epstein-Barr Virus-Associated Post-Transplant Lymphoproliferative Disorders after Hematopoietic Stem Cell Transplantation: Pathogenesis, Risk Factors and Clinical Outcomes.Cancers (Basel). 2020 Feb 1;12(2):328. doi: 10.3390/cancers12020328. Cancers (Basel). 2020. PMID: 32024048 Free PMC article. Review.
-
Plasma Virome of HIV-infected Subjects on Suppressive Antiretroviral Therapy Reveals Association of Differentially Abundant Viruses with Distinct T-cell Phenotypes and Inflammation.Curr Genomics. 2024 Apr 8;25(2):105-119. doi: 10.2174/0113892029279786240111052824. Curr Genomics. 2024. PMID: 38751600 Free PMC article.
-
Epstein-Barr Virus (EBV) Genotypes Associated with the Immunopathological Profile of People Living with HIV-1: Immunological Aspects of Primary EBV Infection.Viruses. 2022 Jan 18;14(2):168. doi: 10.3390/v14020168. Viruses. 2022. PMID: 35215762 Free PMC article.
References
-
- Anselmi A., Vendrame D., Rampon O., Giaquinto C., Zanchetta M, De Rossi A. (2007). Immune reconstitution in human immunodeficiency virus type 1-infected children with different virological responses to anti-retroviral therapy. Clin. Exp. Immunol. 150 442–45010.1111/j.1365-2249.2007.03526.x - DOI - PMC - PubMed
-
- Audigé A., Schlaepfer E., von Wyl V., Miller R. C., Vernazza P., Nadal D., et al. (2010). B cells from HIV-infected patients with primary central nervous system lymphoma display an activated phenotype and have a blunted TNF-α response to TLR9 triggering. AIDS Res. Hum. Retroviruses 26 1063–107410.1089/aid.2009.0288 - DOI - PubMed
-
- Baiocchi R. A., Caligiuri M. A. (1994). Low-dose interleukin 2 prevents the development of Epstein–Barr virus (EBV)-associated lymphoproliferative disease in scid/scid mice reconstituted i.p. with EBV-seropositive human peripheral blood lymphocytes. Proc. Natl. Acad. Sci. U.S.A. 91 5577–5581 10.1073/pnas.91.12.5577 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

