The Effect of Molecular Weight, PK, and Valency on Tumor Biodistribution and Efficacy of Antibody-Based Drugs
- PMID: 24151537
- PMCID: PMC3799199
- DOI: 10.1593/tlo.13409
The Effect of Molecular Weight, PK, and Valency on Tumor Biodistribution and Efficacy of Antibody-Based Drugs
Abstract
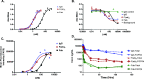
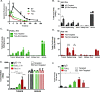
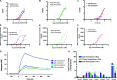
Poor drug delivery and penetration of antibody-mediated therapies pose significant obstacles to effective treatment of solid tumors. This study explored the role of pharmacokinetics, valency, and molecular weight in maximizing drug delivery. Biodistribution of a fibroblast growth factor receptor 4 (FGFR4) targeting CovX-body (an FGFR4-binding peptide covalently linked to a nontargeting IgG scaffold; 150 kDa) and enzymatically generated FGFR4 targeting F(ab)2 (100 kDa) and Fab (50 kDa) fragments was measured. Peak tumor levels were achieved in 1 to 2 hours for Fab and F(ab)2 versus 8 hours for IgG, and the percentage injected dose in tumors was 0.45%, 0.5%, and 2.5%, respectively, compared to 0.3%, 2%, and 6% of their nontargeting controls. To explore the contribution of multivalent binding, homodimeric peptides were conjugated to the different sized scaffolds, creating FGFR4 targeting IgG and F(ab)2 with four peptides and Fab with two peptides. Increased valency resulted in an increase in cell surface binding of the bivalent constructs. There was an inverse relationship between valency and intratumoral drug concentration, consistent with targeted consumption. Immunohistochemical analysis demonstrated increased size and increased cell binding decreased tumor penetration. The binding site barrier hypothesis suggests that limited tumor penetration, as a result of high-affinity binding, could result in decreased efficacy. In our studies, increased target binding translated into superior efficacy of the IgG instead, because of superior inhibition of FGFR4 proliferation pathways and dosing through the binding site barrier. Increasing valency is therefore an effective way to increase the efficacy of antibody-based drugs.
Figures



References
-
- Tunggal JK, Cowan DS, Shaikh H, Tannock IF. Penetration of anticancer drugs through solid tissue: a factor that limits the effectiveness of chemotherapy for solid tumors. Clin Cancer Res. 1999;5:1583–1586. - PubMed
-
- Sedlacek HH, Seemann G, Hoffmann D, Czech J, Lorenz P, Kolar C, Bosslet K. Antibodies as carriers of cytotoxicity. In: Huber H, Queiber W, editors. Contributions to Oncology. Vol. 43. Basel, Switzerland: Karger; 1992. pp. 74–75.
-
- Chaplin DJ, Olive PL, Durand RE. Intermittent blood flow in a murine tumor: radiobiological effects. Cancer Res. 1987;47:597–601. - PubMed
-
- Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure—an obstacle in cancer therapy. Nat Rev Cancer. 2004;4:806–813. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
