(18)F-FDG Is a Surrogate Marker of Therapy Response and Tumor Recovery after Drug Withdrawal during Treatment with a Dual PI3K/mTOR Inhibitor in a Preclinical Model of Cisplatin-Resistant Ovarian Cancer
- PMID: 24151539
- PMCID: PMC3799200
- DOI: 10.1593/tlo.13100
(18)F-FDG Is a Surrogate Marker of Therapy Response and Tumor Recovery after Drug Withdrawal during Treatment with a Dual PI3K/mTOR Inhibitor in a Preclinical Model of Cisplatin-Resistant Ovarian Cancer
Abstract
Aim: Targeting the phosphoinositide 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) pathway is a potential means of overcoming chemoresistance in ovarian cancer. We investigated the capability of (18)F-fluororodeoxyglucose ((18)F-FDG) small-animal positron emission tomography (SA-PET) to predict the effects of a dual PI3K/mTOR inhibitor (BEZ-235) in a cisplatin-resistant ovarian cancer model.
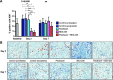
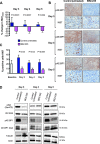
Methods: In a first experiment, nude rats bearing subcutaneous SKOV3 tumors received BEZ-235 for 3 days given alone or after paclitaxel and were compared to controls (either untreated or that were given the excipients of paclitaxel and BEZ-235). SA-PET was performed at baseline, on day 3, and day 7. In a second experiment aiming at further exploring the kinetics of (18)F-FDG tumor uptake during the first 48 hours following drug cessation, untreated controls were compared to rats receiving BEZ-235, which were imaged at baseline, on day 3, on day 4, and on day 5. SA-PET results were compared to cell proliferation assessment (Ki-67), PI3K/mTOR downstream target expression studies (pAKT and phospho-eukaryotic translation initiation factor 4E-binding protein 1), and apoptosis evaluation (cleaved caspase-3).
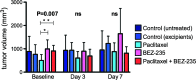
Results: In the first experiment, BEZ-235, compared to untreated controls, induced a marked decrease in (18)F-FDG uptake on day 3, which was correlated to a significant decrease in cell proliferation and to a significant PI3K/mTOR pathway inhibition. No tumor necrosis or apoptosis occurred. Four days following treatment cessation, tumor recovery (in terms of PI3K/mTOR inhibition and cell proliferation) occurred and was identified by (18)F-FDG SA-PET. Paclitaxel plus BEZ-235 showed results similar to BEZ-235 alone. In the second experiment, PI3K/mTOR pathways exhibited partial recovery as early as 24 hours following treatment cessation, but both (18)F-FDG SA-PET and cell proliferation remained unchanged.
Conclusions: (18)F-FDG SA-PET is a surrogate marker of target inhibition during treatment with BEZ-235 and predicts tumor recovery 4 days after drug withdrawal, but not during the first 48 hours following drug cessation, when a lag between PI3K/mTOR pathway recovery and metabolic recovery is observed. (18)F-FDG SA-PET could be used for therapy monitoring of PI3K/mTOR inhibitors, but our results also raise questions regarding the potential impact of the delay between PET imaging and the last drug intake on the accuracy of FDG imaging.
Figures



Similar articles
-
18F-FLT PET as a surrogate marker of drug efficacy during mTOR inhibition by everolimus in a preclinical cisplatin-resistant ovarian tumor model.J Nucl Med. 2010 Oct;51(10):1559-64. doi: 10.2967/jnumed.109.073288. Epub 2010 Sep 16. J Nucl Med. 2010. PMID: 20847160
-
Positron emission tomographic monitoring of dual phosphatidylinositol-3-kinase and mTOR inhibition in anaplastic large cell lymphoma.Onco Targets Ther. 2014 May 23;7:789-98. doi: 10.2147/OTT.S59314. eCollection 2014. Onco Targets Ther. 2014. PMID: 24920919 Free PMC article.
-
[18F]fluorodeoxyglucose positron emission tomography correlates with Akt pathway activity but is not predictive of clinical outcome during mTOR inhibitor therapy.J Clin Oncol. 2009 Jun 1;27(16):2697-704. doi: 10.1200/JCO.2008.18.8383. Epub 2009 Apr 20. J Clin Oncol. 2009. PMID: 19380450 Free PMC article. Clinical Trial.
-
Two hits are better than one: targeting both phosphatidylinositol 3-kinase and mammalian target of rapamycin as a therapeutic strategy for acute leukemia treatment.Oncotarget. 2012 Apr;3(4):371-94. doi: 10.18632/oncotarget.477. Oncotarget. 2012. PMID: 22564882 Free PMC article. Review.
-
Monitoring of anti-cancer treatment with (18)F-FDG and (18)F-FLT PET: a comprehensive review of pre-clinical studies.Am J Nucl Med Mol Imaging. 2015 Oct 12;5(5):431-56. eCollection 2015. Am J Nucl Med Mol Imaging. 2015. PMID: 26550536 Free PMC article. Review.
Cited by
-
Preclinical Evaluation of 18F-ML-10 to Determine Timing of Apoptotic Response to Chemotherapy in Solid Tumors.Mol Imaging. 2017 Jan 1;16:1536012116685941. doi: 10.1177/1536012116685941. Mol Imaging. 2017. PMID: 28654376 Free PMC article.
-
Predicting tumor response and outcome of second-look surgery with 18F-FDG PET/CT: insights from the GINECO CHIVA phase II trial of neoadjuvant chemotherapy plus nintedanib in stage IIIc-IV FIGO ovarian cancer.Eur J Nucl Med Mol Imaging. 2021 Jun;48(6):1998-2008. doi: 10.1007/s00259-020-05092-3. Epub 2020 Nov 21. Eur J Nucl Med Mol Imaging. 2021. PMID: 33221969 Free PMC article.
-
Phase I Study of Apitolisib (GDC-0980), Dual Phosphatidylinositol-3-Kinase and Mammalian Target of Rapamycin Kinase Inhibitor, in Patients with Advanced Solid Tumors.Clin Cancer Res. 2016 Jun 15;22(12):2874-84. doi: 10.1158/1078-0432.CCR-15-2225. Epub 2016 Jan 19. Clin Cancer Res. 2016. PMID: 26787751 Free PMC article. Clinical Trial.
-
Quantifying and correcting for tail vein extravasation in small animal PET scans in cancer research: is there an impact on therapy assessment?EJNMMI Res. 2015 Dec;5(1):61. doi: 10.1186/s13550-015-0141-z. Epub 2015 Nov 5. EJNMMI Res. 2015. PMID: 26543028 Free PMC article.
-
The Emerging Hallmarks of Cancer Metabolism.Cell Metab. 2016 Jan 12;23(1):27-47. doi: 10.1016/j.cmet.2015.12.006. Cell Metab. 2016. PMID: 26771115 Free PMC article. Review.
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. - PubMed
-
- Miller DS, Blessing JA, Krasner CN, Mannel RS, Hanjani P, Pearl ML, Waggoner SE, Boardman CH. Phase II evaluation of pemetrexed in the treatment of recurrent or persistent platinum-resistant ovarian or primary peritoneal carcinoma: a study of the Gynecologic Oncology Group. J Clin Oncol. 2009;27:2686–2691. - PMC - PubMed
-
- Aletti GD, Dowdy SC, Podratz KC, Cliby WA. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. Am J Obstet Gynecol. 2007;197:676–677. - PubMed
-
- Armstrong DK. Relapsed ovarian cancer: challenges and management strategies for a chronic disease. Oncologist 7(suppl. 2002;5):20–28. - PubMed
-
- Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, Lacave AJ. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol. 2001;19:3312–3322. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
