Extending the serum half-life of G-CSF via fusion with the domain III of human serum albumin
- PMID: 24151579
- PMCID: PMC3787585
- DOI: 10.1155/2013/107238
Extending the serum half-life of G-CSF via fusion with the domain III of human serum albumin
Abstract
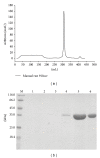
Protein fusion technology is one of the most commonly used methods to extend the half-life of therapeutic proteins. In this study, in order to prolong the half-life of Granulocyte colony stimulating factor (G-CSF), the domain III of human serum albumin (3DHSA) was genetically fused to the N-terminal of G-CSF. The 3DHSA-G-CSF fusion gene was cloned into pPICZ α A along with the open reading frame of the α -factor signal under the control of the AOX1 promoter. The recombinant expression vector was transformed into Pichia pastoris GS115, and the recombinant strains were screened by SDS-PAGE. As expected, the 3DHSA-G-CSF showed high binding affinity with HSA antibody and G-CSF antibody, and the natural N-terminal of 3DHSA was detected by N-terminal sequencing. The bioactivity and pharmacokinetic studies of 3DHSA-G-CSF were respectively determined using neutropenia model mice and human G-CSF ELISA kit. The results demonstrated that 3DHSA-G-CSF has the ability to increase the peripheral white blood cell (WBC) counts of neutropenia model mice, and the half-life of 3DHSA-G-CSF is longer than that of native G-CSF. In conclusion, 3DHSA can be used to extend the half-life of G-CSF.
Figures






References
-
- Nagata S, Tsuchiya M, Asano S. Molecular cloning and expression of cDNA for human granulocyte colony-stimulating factor. Nature. 1986;319(6052):415–418. - PubMed
-
- Sheikh H, Colaco R, Lorigan P, et al. Use of G-CSF during concurrent chemotherapy and thoracic radiotherapy in patients with limited-stage small-cell lung cancer safety data from a phase II trial. Lung Cancer. 2011;74(1):75–79. - PubMed
-
- Meuer K, Pitzer C, Teismann P, et al. Granulocyte-colony stimulating factor is neuroprotective in a model of Parkinson’s disease. Journal of Neurochemistry. 2006;97(3):675–686. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

