Control of perfusable microvascular network morphology using a multiculture microfluidic system
- PMID: 24151838
- PMCID: PMC4074745
- DOI: 10.1089/ten.TEC.2013.0370
Control of perfusable microvascular network morphology using a multiculture microfluidic system
Abstract
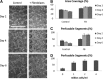
The mechanical and biochemical microenvironment influences the morphological characteristics of microvascular networks (MVNs) formed by endothelial cells (ECs) undergoing the process of vasculogenesis. The objective of this study was to quantify the role of individual factors in determining key network parameters in an effort to construct a set of design principles for engineering vascular networks with prescribed morphologies. To achieve this goal, we developed a multiculture microfluidic platform enabling precise control over paracrine signaling, cell-seeding densities, and hydrogel mechanical properties. Human umbilical vein endothelial cells (HUVECs) were seeded in fibrin gels and cultured alongside human lung fibroblasts (HLFs). The engineered vessels formed in our device contained patent, perfusable lumens. Communication between the two cell types was found to be critical in avoiding network regression and maintaining stable morphology beyond 4 days. The number of branches, average branch length, percent vascularized area, and average vessel diameter were found to depend uniquely on several input parameters. Importantly, multiple inputs were found to control any given output network parameter. For example, the vessel diameter can be decreased either by applying angiogenic growth factors--vascular endothelial growth factor (VEGF) and sphingosine-1-phsophate (S1P)--or by increasing the fibrinogen concentration in the hydrogel. These findings introduce control into the design of MVNs with specified morphological properties for tissue-specific engineering applications.
Figures





References
-
- Kassab G.S.Scaling laws of vascular trees: of form and function. Am J Physiol Heart Circ Physiol 290,H894, 2006 - PubMed
-
- Muschler G.F., Nakamoto C., and Griffith L.G.Engineering Principles of Clinical Cell-Based Tissue Engineering. J Bone Jt Surg 86,1541, 2004 - PubMed
-
- Barber R.W., and Emerson D.R.Biomimetic design of artificial micro-vasculatures for tissue engineering. Altern Lab Anim ATLA 38Suppl 1,67, 2010 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

