Metabolic engineering of biomass for high energy density: oilseed-like triacylglycerol yields from plant leaves
- PMID: 24151938
- PMCID: PMC4285938
- DOI: 10.1111/pbi.12131
Metabolic engineering of biomass for high energy density: oilseed-like triacylglycerol yields from plant leaves
Abstract
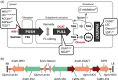
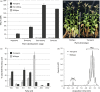
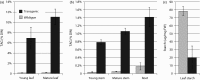
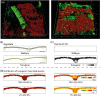
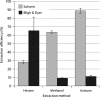
High biomass crops have recently attracted significant attention as an alternative platform for the renewable production of high energy storage lipids such as triacylglycerol (TAG). While TAG typically accumulates in seeds as storage compounds fuelling subsequent germination, levels in vegetative tissues are generally low. Here, we report the accumulation of more than 15% TAG (17.7% total lipids) by dry weight in Nicotiana tabacum (tobacco) leaves by the co-expression of three genes involved in different aspects of TAG production without severely impacting plant development. These yields far exceed the levels found in wild-type leaf tissue as well as previously reported engineered TAG yields in vegetative tissues of Arabidopsis thaliana and N. tabacum. When translated to a high biomass crop, the current levels would translate to an oil yield per hectare that exceeds those of most cultivated oilseed crops. Confocal fluorescence microscopy and mass spectrometry imaging confirmed the accumulation of TAG within leaf mesophyll cells. In addition, we explored the applicability of several existing oil-processing methods using fresh leaf tissue. Our results demonstrate the technical feasibility of a vegetative plant oil production platform and provide for a step change in the bioenergy landscape, opening new prospects for sustainable food, high energy forage, biofuel and biomaterial applications.
Keywords: DGAT1; Nicotiana tabacum; WRI1; leaf; oleosin; triacylglycerol.
© 2013 CSIRO. Plant Biotechnology Journal published by Society for Experimental Biology, Association of Applied Biologists and John Wiley & Sons Ltd.
Figures
References
-
- Andrianov V, Borisjuk N, Pogrebnyak N, Brinker A, Dixon J, Spitsin S, Flynn J, Matyszczuk P, Andryszak K, Laurelli M, Golovkin M, Koprowski H. Tobacco as a production platform for biofuel: overexpression of Arabidopsis DGAT and LEC2 genes increases accumulation and shifts the composition of lipids in green biomass. Plant Biotechnol. J. 2010;8:277–287. - PubMed
-
- Bouvier-Nave P, Benveniste P, Oelkers P, Sturley SL, Schaller H. Expression in yeast and tobacco of plant cDNAs encoding acyl CoA:diacylglycerol acyltransferases. Eur. J. Biochem. 2000;267:85–96. - PubMed
-
- Cernac A, Benning C. WRINKLED1 encodes an AP2/EREB domain protein involved in the control of storage compound biosynthesis in Arabidopsis. Plant J. 2004;40:575–585. - PubMed
-
- Chapman K, Dyer JM, Mullen RT. Why don't plant leaves get fat? Plant Sci. 2013;207:128–134. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

