Pharmacokinetics and pharmacodynamics of intravenous and inhaled fluticasone furoate in healthy Caucasian and East Asian subjects
- PMID: 24152086
- PMCID: PMC4004401
- DOI: 10.1111/bcp.12263
Pharmacokinetics and pharmacodynamics of intravenous and inhaled fluticasone furoate in healthy Caucasian and East Asian subjects
Abstract
Aim: The aim of the study was to evaluate the pharmacokinetics (PK) of inhaled and intravenous (i.v.) fluticasone furoate (FF) in healthy Caucasian, Chinese, Japanese and Korean subjects.
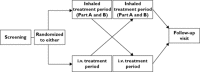
Method: This was an open label, randomized, two way crossover study in healthy Caucasian, Chinese, Japanese and Korean subjects (n = 20 per group). Inhaled FF (200 μg for 7 days, then 800 μg for 7 days from a dry powder inhaler [DPI]) was administered in one treatment period and i.v.FF (250 μg infusion) in the other. FF PK and serum cortisol (inhaled 200 μg only) were compared between the ethnic groups using analysis of variance. P450 CYP3A4 activity and safety were also assessed.
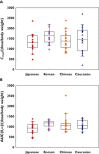
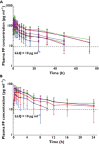
Results: Ethnic differences in i.v. FF PK were accounted for by body weight differences. CYP3A4 activity was similar across the groups. Higher FF systemic exposure was seen following inhaled dosing in Chinese, Japanese and Korean subjects compared with Caucasian subjects. Absolute bioavailability was greater (36%-55%) in all East Asian groups than for Caucasian subjects following inhaled FF 800 μg. Deconvolution analysis suggested inhaled FF resided in the lung of East Asian subjects longer than for Caucasians (time for 90% to be absorbed [t90]: 29.1-30.8 h vs. 21.4 h). In vitro simulation method predicted comparable delivered lung dose across ethnic groups. Serum cortisol weighted mean was similar between Caucasians and Chinese or Koreans, while in Japanese was on average 22% lower than in Caucasians. All FF treatments were safe and well tolerated.
Conclusion: Modestly higher (<50%) FF systemic exposure seen in East Asian subjects following inhaled dosing was not associated with a clinically significant effect on serum cortisol, suggesting that a clinical dose adjustment in East Asian subjects is not required.
Keywords: Caucasian; East Asian; fluticasone furoate; healthy subjects; pharmacodynamics; pharmacokinetics.
© 2013 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of The British Pharmacological Society.
Figures
 ) Caucasian (repeat), (
) Caucasian (repeat), ( ) Caucasian (single), (
) Caucasian (single), ( ) Chinese (repeat), (
) Chinese (repeat), ( ) Chinese (single), (
) Chinese (single), ( ) Japanese (repeat), (
) Japanese (repeat), ( ) Japanese (single), (
) Japanese (single), ( ) Korean (repeat), and (
) Korean (repeat), and ( ) Korean (single). 3B: (
) Korean (single). 3B: ( ) Caucasian, (
) Caucasian, ( ) Chinese, (
) Chinese, ( ) Japanese and (
) Japanese and ( ) Korean
) Korean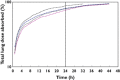
 ) Caucasian, (
) Caucasian, ( ) Chinese, (
) Chinese, ( ) Japanese, and (
) Japanese, and ( ) Korean
) Korean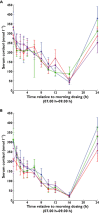
 ) Caucasian, (
) Caucasian, ( ) Chinese, (
) Chinese, ( ) Japanese and (
) Japanese and ( ) Korean
) KoreanSimilar articles
-
Comparative clinical pharmacology of mometasone furoate, fluticasone propionate and fluticasone furoate.Pulm Pharmacol Ther. 2022 Dec;77:102171. doi: 10.1016/j.pupt.2022.102171. Epub 2022 Oct 13. Pulm Pharmacol Ther. 2022. PMID: 36243386 Clinical Trial.
-
Pharmacodynamics and pharmacokinetics of fluticasone furoate/vilanterol in healthy Chinese subjects.Pharmacotherapy. 2015 Jun;35(6):586-99. doi: 10.1002/phar.1598. Epub 2015 Jun 9. Pharmacotherapy. 2015. PMID: 26059225 Free PMC article. Clinical Trial.
-
The pharmacodynamics, pharmacokinetics, safety and tolerability of inhaled fluticasone furoate and vilanterol administered alone or simultaneously as fluticasone furoate/vilanterol.Clin Pharmacol Drug Dev. 2015 Jan;4(1):2-11. doi: 10.1002/cpdd.160. Epub 2014 Dec 5. Clin Pharmacol Drug Dev. 2015. PMID: 27127998 Clinical Trial.
-
Pharmacodynamic and pharmacokinetic assessment of fluticasone furoate + vilanterol for the treatment of asthma.Expert Opin Drug Metab Toxicol. 2016 Jul;12(7):813-22. doi: 10.1080/17425255.2016.1192125. Epub 2016 Jun 9. Expert Opin Drug Metab Toxicol. 2016. PMID: 27253498 Review.
-
Fluticasone furoate and vilanterol inhalation powder for the treatment of chronic obstructive pulmonary disease.Expert Rev Respir Med. 2015 Feb;9(1):5-12. doi: 10.1586/17476348.2015.986468. Epub 2014 Dec 6. Expert Rev Respir Med. 2015. PMID: 25482512 Review.
Cited by
-
Profile of fluticasone furoate/vilanterol dry powder inhaler combination therapy as a potential treatment for COPD.Int J Chron Obstruct Pulmon Dis. 2014 Feb 24;9:249-56. doi: 10.2147/COPD.S32604. eCollection 2014. Int J Chron Obstruct Pulmon Dis. 2014. PMID: 24596460 Free PMC article. Review.
-
Population Pharmacokinetics of Inhaled Fluticasone Furoate and Vilanterol in Subjects with Chronic Obstructive Pulmonary Disease.Eur J Drug Metab Pharmacokinet. 2016 Dec;41(6):743-758. doi: 10.1007/s13318-015-0303-4. Eur J Drug Metab Pharmacokinet. 2016. PMID: 26474864 Free PMC article. Clinical Trial.
-
Ethnic sensitivity assessment of fluticasone furoate/vilanterol in East Asian asthma patients from randomized double-blind multicentre Phase IIb/III trials.BMC Pulm Med. 2015 Dec 24;15:165. doi: 10.1186/s12890-015-0159-z. BMC Pulm Med. 2015. PMID: 26704701 Free PMC article. Clinical Trial.
-
Pharmacokinetics and tolerability of inhaled umeclidinium and vilanterol alone and in combination in healthy Chinese subjects: a randomized, open-label, crossover trial.PLoS One. 2015 Mar 27;10(3):e0121264. doi: 10.1371/journal.pone.0121264. eCollection 2015. PLoS One. 2015. PMID: 25816315 Free PMC article. Clinical Trial.
-
Model-based evaluation of pulmonary pharmacokinetics in asthmatic and COPD patients after oral olodaterol inhalation.Br J Clin Pharmacol. 2016 Sep;82(3):739-53. doi: 10.1111/bcp.12999. Epub 2016 Jun 23. Br J Clin Pharmacol. 2016. PMID: 27145733 Free PMC article. Clinical Trial.
References
-
- Lötvall J, Bakke PS, Bjermer L, Steinshamn S, Scott-Wilson C, Crim C, Sanford L, Haumann B. Efficacy and safety of 4 weeks' treatment with combined fluticasone furoate/vilanterol in a single inhaler given once daily in COPD: a placebo-controlled randomised trial. BMJ Open. 2012;2:e000370. - PMC - PubMed
-
- Kempsford RD, Allen A, Bareille P, Bishop H, Hamilton M, Cheesbrough A. The safety, tolerability, pharmacodynamics and pharmacokinetics of inhaled fluticasone furoate (FF) and vilanterol (VI) are unaffected by administration in combination. ERJ. 2011;38(Suppl. 55):138s.
-
- Bernstein DI, Allen DB. Evaluation of tests of hypothalamic-pituitary-adrenal axis function used to measure effects of inhaled corticosteroids. Ann Allergy Asthma Immunol. 2007;98:118–127. - PubMed
-
- Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysis. Chest. 2004;125:2309–2321. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

