Fluorescent biphenyl derivatives of phenylalanine suitable for protein modification
- PMID: 24152169
- PMCID: PMC3875372
- DOI: 10.1021/bi401275v
Fluorescent biphenyl derivatives of phenylalanine suitable for protein modification
Abstract
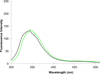
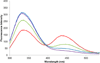
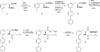
In a recent study, we demonstrated that structurally compact fluorophores incorporated into the side chains of amino acids could be introduced into dihydrofolate reductase from Escherichia coli (ecDHFR) with minimal disruption of protein structure or function, even when the site of incorporation was within a folded region of the protein. The modified proteins could be employed for FRET measurements, providing sensitive monitors of changes in protein conformation. The very favorable results achieved in that study encouraged us to prepare additional fluorescent amino acids of potential utility for studying protein dynamics. Presently, we describe the synthesis and photophysical characterization of four positional isomers of biphenyl-phenylalanine, all of which were found to exhibit potentially useful fluorescent properties. All four phenylalanine derivatives were used to activate suppressor tRNA transcripts and incorporated into multiple positions of ecDHFR. All phenylalanine derivatives were incorporated with good efficiency into position 16 of ecDHFR and afforded modified proteins that consumed NADPH at rates up to about twice the rate measured for wild type. This phenomenon has been noted on a number of occasions previously and shown to be due to an increase in the off-rate of tetrahydrofolate from the enzyme, altering a step that is normally rate limiting. When introduced into sterically accessible position 49, the four phenylalanine derivatives afforded DHFRs having catalytic function comparable to wild type. The four phenylalanine derivatives were also introduced into position 115 of ecDHFR, which is known to be a folded region of the protein less tolerant of structural alteration. As anticipated, significant differences were noted in the catalytic efficiencies of the derived proteins. The ability of two of the sizable biphenyl-phenylalanine derivatives to be accommodated at position 115 with minimal perturbation of DHFR function is attributed to rotational flexibility about the biphenyl bonds.
Figures




Similar articles
-
Detection of dihydrofolate reductase conformational change by FRET using two fluorescent amino acids.J Am Chem Soc. 2013 Sep 4;135(35):12924-7. doi: 10.1021/ja403007r. Epub 2013 Aug 22. J Am Chem Soc. 2013. PMID: 23941571 Free PMC article.
-
Conformation coupled enzyme catalysis: single-molecule and transient kinetics investigation of dihydrofolate reductase.Biochemistry. 2005 Dec 27;44(51):16835-43. doi: 10.1021/bi051378i. Biochemistry. 2005. PMID: 16363797
-
The coordination of the isomerization of a conserved non-prolyl cis peptide bond with the rate-limiting steps in the folding of dihydrofolate reductase.J Mol Biol. 2003 Feb 14;326(2):569-83. doi: 10.1016/s0022-2836(02)01444-4. J Mol Biol. 2003. PMID: 12559923
-
Environmental Adaptation of Dihydrofolate Reductase from Deep-Sea Bacteria.Subcell Biochem. 2015;72:423-42. doi: 10.1007/978-94-017-9918-8_21. Subcell Biochem. 2015. PMID: 26174394 Review.
-
Chemical space of Escherichia coli dihydrofolate reductase inhibitors: New approaches for discovering novel drugs for old bugs.Med Res Rev. 2019 Mar;39(2):684-705. doi: 10.1002/med.21538. Epub 2018 Sep 7. Med Res Rev. 2019. PMID: 30192413 Free PMC article. Review.
Cited by
-
Tyrosine-derived stimuli responsive, fluorescent amino acids.Chem Sci. 2015 Feb 1;6(2):1150-1158. doi: 10.1039/c4sc02753a. Epub 2014 Oct 31. Chem Sci. 2015. PMID: 29560202 Free PMC article.
-
Expedient discovery of fluorogenic amino acid-based probes via one-pot palladium-catalysed arylation of tyrosine.Chem Sci. 2025 Jan 23;16(8):3490-3497. doi: 10.1039/d5sc00020c. eCollection 2025 Feb 19. Chem Sci. 2025. PMID: 39886437 Free PMC article.
-
Tryptophan-based fluorophores for studying protein conformational changes.Bioorg Med Chem. 2014 Nov 1;22(21):5924-34. doi: 10.1016/j.bmc.2014.09.015. Epub 2014 Sep 16. Bioorg Med Chem. 2014. PMID: 25284250 Free PMC article.
-
Fluorescent amino acids as versatile building blocks for chemical biology.Nat Rev Chem. 2020 Jun;4(6):275-290. doi: 10.1038/s41570-020-0186-z. Epub 2020 May 13. Nat Rev Chem. 2020. PMID: 37127957 Review.
-
Effects of Distal Mutations on Ligand-Binding Affinity in E. coli Dihydrofolate Reductase.ACS Omega. 2021 Oct 1;6(40):26065-26076. doi: 10.1021/acsomega.1c02995. eCollection 2021 Oct 12. ACS Omega. 2021. PMID: 34660967 Free PMC article.
References
-
- Tsien RY. The green fluorescent protein. Annu. Rev. Biochem. 1998;67:509–544. - PubMed
-
- Selvin PR. The renaissance of fluorescence resonance energy transfer. Nat. Struct. Biol. 2000;7:730–734. - PubMed
-
- Phillips SR, Wilson LJ, Borkman RF. Acrylamide and iodide fluorescence quenching as a structural probe of tryptophan microenvironment in bovine lens crystallins. Curr. Eye Res. 1986;5:611–619. - PubMed
-
- Zhang P, Beck T, Tan W. Design of a molecular beacon DNA probe with two fluorophores. Angew. Chem. Int. Ed. 2001;40:402–405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

