Translocation of cationic amphipathic peptides across the membranes of pure phospholipid giant vesicles
- PMID: 24152283
- PMCID: PMC3922827
- DOI: 10.1021/ja407451c
Translocation of cationic amphipathic peptides across the membranes of pure phospholipid giant vesicles
Abstract
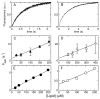
The ability of amphipathic polypeptides with substantial net positive charges to translocate across lipid membranes is a fundamental problem in physical biochemistry. These peptides should not passively cross the bilayer nonpolar region, but they do. Here we present a method to measure peptide translocation and test it on three representative membrane-active peptides. In samples of giant unilamellar vesicles (GUVs) prepared by electroformation, some GUVs enclose inner vesicles. When these GUVs are added to a peptide solution containing a membrane-impermeant fluorescent dye (carboxyfluorescein), the peptide permeabilizes the outer membrane, and dye enters the outer GUV, which then exhibits green fluorescence. The inner vesicles remain dark if the peptide does not cross the outer membrane. However, if the peptide translocates, it permeabilizes the inner vesicles as well, which then show fluorescence. We also measure translocation, simultaneously on the same GUV, by the appearance of fluorescently labeled peptides on the inner vesicle membranes. All three peptides examined are able to translocate, but to different extents. Peptides with smaller Gibbs energies of insertion into the membrane translocate more easily. Further, translocation and influx occur broadly over the same period, but with very different kinetics. Translocation across the outer membrane follows approximately an exponential rise, with a characteristic time of 10 min. Influx occurs more abruptly. In the outer vesicle, influx happens before most of the translocation. However, some peptides cross the membrane before any influx is observed. In the inner vesicles, influx occurs abruptly sometime during peptide translocation across the membrane of the outer vesicle.
Conflict of interest statement
The authors declare no competing financial interests.
Figures







References
-
- Langel U. Handbook of Cell Penetrating Peptides. 2. CRC Press; Boca Raton, FL: 2006.
-
- Derossi D, Calvet S, Trembleau A, Brunissen A, Chassaing G, Prochiantz A. J Biol Chem. 1996;271:18188–18193. - PubMed
-
- Green M, Weston PM. Cell. 1988;55:1179–1188. - PubMed
-
- Frankel AD, Pabo CO. Cell. 1988;55:1189–1193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

