Epitope mapping of inhibitory antibodies targeting the C2 domain of coagulation factor VIII by hydrogen-deuterium exchange mass spectrometry
- PMID: 24152306
- PMCID: PMC3947443
- DOI: 10.1111/jth.12433
Epitope mapping of inhibitory antibodies targeting the C2 domain of coagulation factor VIII by hydrogen-deuterium exchange mass spectrometry
Abstract
Background: The development of anti-factor VIII antibodies (inhibitors) is a significant complication in the management of patients with hemophilia A, leading to significant increases in morbidity and treatment cost. Using a panel of mAbs against different epitopes on FVIII, we have recently shown that epitope specificity, inhibitor kinetics and time to maximum inhibition are more important than inhibitor titer in predicting responses to FVIII and the combination of FVIII and recombinant FVIIa. In particular, a subset of high-titer inhibitors responded to high-dose FVIII, which would not be predicted on the basis of their inhibitor titer alone. Thus, the ability to quickly map the epitope spectrum of patient plasma with a clinically feasible assay may fundamentally change how clinicians approach the treatment of high-titer inhibitor patients.
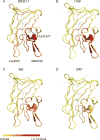
Objectives: To map the epitopes of anti-FVIII mAbs, three of which are classic inhibitors and one of which is a non-classic inhibitor, by the use of hydrogen-deuterium exchange coupled with mass spectrometry (HDX-MS).
Methods: The binding epitopes of four mAbs targeting the FVIII C2 domain were mapped with HDX-MS.
Results: The epitopes determined with HDX-MS are consistent with those obtained earlier through structural characterization and antibody competition assays. In addition, classic and non-classic inhibitor epitopes could be distinguished by the use of a limited subset of C2 domain-derived peptic fragments.
Conclusion: Our results demonstrate the effectiveness and robustness of the HDX-MS method for epitope mapping, and suggest a potential role of rapid mapping of FVIII inhibitor epitopes in facilitating individualized treatment of inhibitor patients.
Keywords: antigen-antibody complex; epitope mapping; factor VIII; hemophilia A; hydrogen deuterium exchange measurement.
© 2013 International Society on Thrombosis and Haemostasis.
Conflict of interest statement
Figures





Similar articles
-
High-affinity, noninhibitory pathogenic C1 domain antibodies are present in patients with hemophilia A and inhibitors.Blood. 2016 Oct 20;128(16):2055-2067. doi: 10.1182/blood-2016-02-701805. Epub 2016 Jul 5. Blood. 2016. PMID: 27381905 Free PMC article.
-
Potentiation of thrombin generation in hemophilia A plasma by coagulation factor VIII and characterization of antibody-specific inhibition.PLoS One. 2012;7(10):e48172. doi: 10.1371/journal.pone.0048172. Epub 2012 Oct 29. PLoS One. 2012. PMID: 23144741 Free PMC article.
-
Some factor VIII inhibitor antibodies recognize a common epitope corresponding to C2 domain amino acids 2248 through 2312, which overlap a phospholipid-binding site.Blood. 1995 Sep 1;86(5):1811-9. Blood. 1995. PMID: 7544643
-
Analysis of factor VIII inhibitors using hybrid human/porcine factor VIII.Thromb Haemost. 1997 Jul;78(1):647-51. Thromb Haemost. 1997. PMID: 9198232 Review.
-
Computational Structure Prediction for Antibody-Antigen Complexes From Hydrogen-Deuterium Exchange Mass Spectrometry: Challenges and Outlook.Front Immunol. 2022 May 26;13:859964. doi: 10.3389/fimmu.2022.859964. eCollection 2022. Front Immunol. 2022. PMID: 35720345 Free PMC article. Review.
Cited by
-
Current approaches to fine mapping of antigen-antibody interactions.Immunology. 2014 Aug;142(4):526-35. doi: 10.1111/imm.12284. Immunology. 2014. PMID: 24635566 Free PMC article. Review.
-
Structural mass spectrometry decodes domain interaction and dynamics of the full-length Human Histone Deacetylase 2.Biochim Biophys Acta Proteins Proteom. 2022 Mar 1;1870(3):140759. doi: 10.1016/j.bbapap.2022.140759. Epub 2022 Jan 18. Biochim Biophys Acta Proteins Proteom. 2022. PMID: 35051665 Free PMC article.
-
High Resolution Mapping of Bactericidal Monoclonal Antibody Binding Epitopes on Staphylococcus aureus Antigen MntC.PLoS Pathog. 2016 Sep 30;12(9):e1005908. doi: 10.1371/journal.ppat.1005908. eCollection 2016 Sep. PLoS Pathog. 2016. PMID: 27689696 Free PMC article.
-
Engineering less immunogenic and antigenic FVIII proteins.Cell Immunol. 2016 Mar;301:12-7. doi: 10.1016/j.cellimm.2015.10.008. Epub 2015 Nov 2. Cell Immunol. 2016. PMID: 26566286 Free PMC article. Review.
-
High-affinity, noninhibitory pathogenic C1 domain antibodies are present in patients with hemophilia A and inhibitors.Blood. 2016 Oct 20;128(16):2055-2067. doi: 10.1182/blood-2016-02-701805. Epub 2016 Jul 5. Blood. 2016. PMID: 27381905 Free PMC article.
References
-
- Scharrer I, Bray GL, Neutzling O. Incidence of inhibitors in haemophilia A patients--a review of recent studies of recombinant and plasma-derived factor VIII concentrates. Haemophilia. 1999;5:145–154. - PubMed
-
- Hoots WK. The future of plasma-derived clotting factor concentrates. Haemophilia. 2001;7(Suppl 1):4–9. - PubMed
-
- Bray GL, Gomperts ED, Courter S, Gruppo R, Gordon EM, Manco-Johnson M, Shapiro A, Scheibel E, White G, 3rd, Lee M. A multicenter study of recombinant factor VIII (recombinate): safety, efficacy, and inhibitor risk in previously untreated patients with hemophilia A. The Recombinate Study Group. Blood. 1994;83:2428–2435. - PubMed
-
- Lusher JM, Lee CA, Kessler CM, Bedrosian CL. The safety and efficacy of B-domain deleted recombinant factor VIII concentrate in patients with severe haemophilia A. Haemophilia. 2003;9:38–49. - PubMed
-
- Scandella D, Gilbert GE, Shima M, Nakai H, Eagleson C, Felch M, Prescott R, Rajalakshmi KJ, Hoyer LW, Saenko E. Some factor VIII inhibitor antibodies recognize a common epitope corresponding to C2 domain amino acids 2248 through 2312, which overlap a phospholipid-binding site. Blood. 1995;86:1811–1819. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

