Chimeras of sperm PLCζ reveal disparate protein domain functions in the generation of intracellular Ca2+ oscillations in mammalian eggs at fertilization
- PMID: 24152875
- PMCID: PMC3843027
- DOI: 10.1093/molehr/gat070
Chimeras of sperm PLCζ reveal disparate protein domain functions in the generation of intracellular Ca2+ oscillations in mammalian eggs at fertilization
Abstract
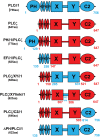
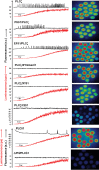
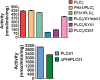
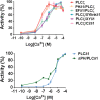
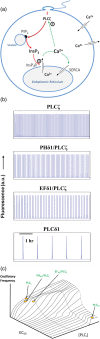
Phospholipase C-zeta (PLCζ) is a sperm-specific protein believed to cause Ca(2+) oscillations and egg activation during mammalian fertilization. PLCζ is very similar to the somatic PLCδ1 isoform but is far more potent in mobilizing Ca(2+) in eggs. To investigate how discrete protein domains contribute to Ca(2+) release, we assessed the function of a series of PLCζ/PLCδ1 chimeras. We examined their ability to cause Ca(2+) oscillations in mouse eggs, enzymatic properties using in vitro phosphatidylinositol 4,5-bisphosphate (PIP2) hydrolysis and their binding to PIP2 and PI(3)P with a liposome interaction assay. Most chimeras hydrolyzed PIP2 with no major differences in Ca(2+) sensitivity and enzyme kinetics. Insertion of a PH domain or replacement of the PLCζ EF hands domain had no deleterious effect on Ca(2+) oscillations. In contrast, replacement of either XY-linker or C2 domain of PLCζ completely abolished Ca(2+) releasing activity. Notably, chimeras containing the PLCζ XY-linker bound to PIP2-containing liposomes, while chimeras containing the PLCζ C2 domain exhibited PI(3)P binding. Our data suggest that the EF hands are not solely responsible for the nanomolar Ca(2+) sensitivity of PLCζ and that membrane PIP2 binding involves the C2 domain and XY-linker of PLCζ. To investigate the relationship between PLC enzymatic properties and Ca(2+) oscillations in eggs, we have developed a mathematical model that incorporates Ca(2+)-dependent InsP3 generation by the PLC chimeras and their levels of intracellular expression. These numerical simulations can for the first time predict the empirical variability in onset and frequency of Ca(2+) oscillatory activity associated with specific PLC variants.
Keywords: sperm, PLC-zeta, calcium oscillations, egg activation, fertilization.
Figures


Similar articles
-
Essential Role of the EF-hand Domain in Targeting Sperm Phospholipase Cζ to Membrane Phosphatidylinositol 4,5-Bisphosphate (PIP2).J Biol Chem. 2015 Dec 4;290(49):29519-30. doi: 10.1074/jbc.M115.658443. Epub 2015 Oct 1. J Biol Chem. 2015. PMID: 26429913 Free PMC article.
-
Role of phospholipase C-zeta domains in Ca2+-dependent phosphatidylinositol 4,5-bisphosphate hydrolysis and cytoplasmic Ca2+ oscillations.J Biol Chem. 2005 Sep 2;280(35):31011-8. doi: 10.1074/jbc.M500629200. Epub 2005 Jul 6. J Biol Chem. 2005. PMID: 16000311
-
The sperm phospholipase C-ζ and Ca2+ signalling at fertilization in mammals.Biochem Soc Trans. 2016 Feb;44(1):267-72. doi: 10.1042/BST20150221. Biochem Soc Trans. 2016. PMID: 26862214 Review.
-
Recombinant phospholipase Czeta has high Ca2+ sensitivity and induces Ca2+ oscillations in mouse eggs.J Biol Chem. 2004 Mar 12;279(11):10408-12. doi: 10.1074/jbc.M313801200. Epub 2003 Dec 29. J Biol Chem. 2004. PMID: 14701816
-
Novel signalling mechanism and clinical applications of sperm-specific PLCζ.Biochem Soc Trans. 2015 Jun;43(3):371-6. doi: 10.1042/BST20140291. Biochem Soc Trans. 2015. PMID: 26009178 Review.
Cited by
-
PLCζ Induced Ca2+ Oscillations in Mouse Eggs Involve a Positive Feedback Cycle of Ca2+ Induced InsP3 Formation From Cytoplasmic PIP2.Front Cell Dev Biol. 2018 Apr 3;6:36. doi: 10.3389/fcell.2018.00036. eCollection 2018. Front Cell Dev Biol. 2018. PMID: 29666796 Free PMC article.
-
Sperm-specific post-acrosomal WW-domain binding protein (PAWP) does not cause Ca2+ release in mouse oocytes.Mol Hum Reprod. 2014 Oct;20(10):938-47. doi: 10.1093/molehr/gau056. Epub 2014 Jul 23. Mol Hum Reprod. 2014. PMID: 25057041 Free PMC article.
-
Human PLCζ exhibits superior fertilization potency over mouse PLCζ in triggering the Ca(2+) oscillations required for mammalian oocyte activation.Mol Hum Reprod. 2014 Jun;20(6):489-98. doi: 10.1093/molehr/gau011. Epub 2014 Jan 29. Mol Hum Reprod. 2014. PMID: 24478462 Free PMC article.
-
Phospholipase C.Adv Exp Med Biol. 2020;1131:215-242. doi: 10.1007/978-3-030-12457-1_9. Adv Exp Med Biol. 2020. PMID: 31646512 Free PMC article. Review.
-
Calcium influx and sperm-evoked calcium responses during oocyte maturation and egg activation.Oncotarget. 2017 Jul 29;8(51):89375-89390. doi: 10.18632/oncotarget.19679. eCollection 2017 Oct 24. Oncotarget. 2017. PMID: 29179526 Free PMC article. Review.
References
-
- Atri A, Amundson J, Clapham D, Sneyd J. A single-pool model for intracellular calcium oscillations and waves in the Xenopus laevis oocyte. Biophys J. 1993;65:1727–1739. doi:10.1016/S0006-3495(93)81191-3. - DOI - PMC - PubMed
-
- Bae YS, Cantley LG, Chen CS, Kim SR, Kwon KS, Rhee SG. Activation of phospholipase C-gamma by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:4465–4469. doi:10.1074/jbc.273.8.4465. - DOI - PubMed
-
- Cox LJ, Larman MG, Saunders CM, Hashimoto K, Swann K, Lai FA. Sperm phospholipase Czeta from humans and cynomolgus monkeys triggers Ca2+ oscillations, activation and development of mouse oocytes. Reproduction. 2002;124:611–623. doi:10.1530/rep.0.1240611. - DOI - PubMed
-
- Dıaz J, Pastor N, Martinez-Mekler G. Role of a spatial distribution of IP3 receptors in the Ca2+ dynamics of the Xenopus embryo at the mid-blastula transition stage. Devel Dyn. 2005;232:301–312. doi:10.1002/dvdy.20238. - DOI - PubMed
-
- Dupont G, Heytens E, Leybaert L. Oscillatory Ca2+ dynamics and cell cycle resumption at fertilization in mammals: a modelling approach. Int J Dev Biol. 2010;54:655–665. doi:10.1387/ijdb.082845gd. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

