Real-time reverse transcription-PCR assay panel for Middle East respiratory syndrome coronavirus
- PMID: 24153118
- PMCID: PMC3911421
- DOI: 10.1128/JCM.02533-13
Real-time reverse transcription-PCR assay panel for Middle East respiratory syndrome coronavirus
Abstract
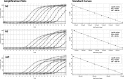
A new human coronavirus (CoV), subsequently named Middle East respiratory syndrome (MERS)-CoV, was first reported in Saudi Arabia in September 2012. In response, we developed two real-time reverse transcription-PCR (rRT-PCR) assays targeting the MERS-CoV nucleocapsid (N) gene and evaluated these assays as a panel with a previously published assay targeting the region upstream of the MERS-CoV envelope gene (upE) for the detection and confirmation of MERS-CoV infection. All assays detected ≤10 copies/reaction of quantified RNA transcripts, with a linear dynamic range of 8 log units and 1.3 × 10(-3) 50% tissue culture infective doses (TCID50)/ml of cultured MERS-CoV per reaction. All assays performed comparably with respiratory, serum, and stool specimens spiked with cultured virus. No false-positive amplifications were obtained with other human coronaviruses or common respiratory viral pathogens or with 336 diverse clinical specimens from non-MERS-CoV cases; specimens from two confirmed MERS-CoV cases were positive with all assay signatures. In June 2012, the U.S. Food and Drug Administration authorized emergency use of the rRT-PCR assay panel as an in vitro diagnostic test for MERS-CoV. A kit consisting of the three assay signatures and a positive control was assembled and distributed to public health laboratories in the United States and internationally to support MERS-CoV surveillance and public health responses.
Figures
References
-
- van Boheemen S, de Graaf M, Lauber C, Bestebroer TM, Raj VS, Zaki AM, Osterhaus AD, Haagmans BL, Gorbalenya AE, Snijder EJ, Fouchier RA. 2012. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. mBio 3:e00473-12. 10.1128/mBio.00473-12 - DOI - PMC - PubMed
-
- de Groot RJ, Baker SC, Baric RS, Brown CS, Drosten C, Enjuanes L, Fouchier RA, Galiano M, Gorbalenya AE, Memish ZA, Perlman S, Poon LL, Snijder EJ, Stephens GM, Woo PC, Zaki AM, Zambon M, Ziebuhr J. 2013. Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. J. Virol. 87:7790–7792. 10.1128/JVI.01244-13 - DOI - PMC - PubMed
-
- Corman VM, Eckerle I, Bleicker T, Zaki A, Landt O, Eschbach-Bludau M, van Boheemen S, Gopal R, Ballhause M, Bestebroer TM, Muth D, Muller MA, Drexler JF, Zambon M, Osterhaus AD, Fouchier RM, Drosten C. 2012. Detection of a novel human coronavirus by real-time reverse-transcription polymerase chain reaction. Euro Surveill. 17:20285 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20285 - PubMed
-
- Corman VM, Muller MA, Costabel U, Timm J, Binger T, Meyer B, Kreher P, Lattwein E, Eschbach-Bludau M, Nitsche A, Bleicker T, Landt O, Schweiger B, Drexler JF, Osterhaus AD, Haagmans BL, Dittmer U, Bonin F, Wolff T, Drosten C. 2012. Assays for laboratory confirmation of novel human coronavirus (hCoV-EMC) infections. Euro Surveill. 17:20334 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20334 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

