Barriers to study enrollment in patients with advanced cancer referred to a phase I clinical trials unit
- PMID: 24153239
- PMCID: PMC3868426
- DOI: 10.1634/theoncologist.2013-0202
Barriers to study enrollment in patients with advanced cancer referred to a phase I clinical trials unit
Abstract
We conducted this retrospective study to identify reasons that patients referred to a phase I clinical trial failed to enroll or delayed enrollment onto the trial.
Materials and methods: Outcome analyses were conducted independently on data collected from electronic medical records of two sets of consecutive patients referred to a phase I clinical trial facility at MD Anderson Cancer Center. Data from the first set of 300 patients were used to determine relevant variables affecting enrollment; data from the second set of 957 patients were then analyzed for these variables.
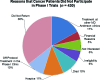
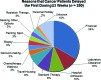
Results: Results from the two sets of patients were similar. Approximately 55% of patients were enrolled in a phase I trial. Patients referred from within MD Anderson were more likely to be enrolled than patients seen originally outside the institution (p = .006); black patients were more likely than white patients to enroll (69% vs. 43%; p = .04). The median interval from the initial visit to initiation of treatments was 19 days. Major reasons for failure to enroll included failure to return to the clinic (36%), opting for treatment in another clinic (17%), hospice referral (11%), early death (10%), and lack of financial clearance (5%). Treatment was delayed for three weeks or more in 250 patients; in 85 patients (34%), the delay was caused by financial and insurance issues.
Conclusion: Failure to return to the clinic, pursuit of other therapy, and rapid deterioration were the major reasons for failure to enroll; lengthy financial clearance was the most common reason for delayed enrollment onto a phase I trial.
Keywords: Barrier; Consults; Enrollment; New patients; Patient referral; Phase I trial.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
References
-
- Kurzrock R, Benjamin RS. Risks and benefits of phase 1 oncology trials, revisited. N Engl J Med. 2005;352:930–932. - PubMed
-
- Fu S, Hong DS, Naing A, et al. Outcome analyses after the first admission to an intensive care unit in patients with advanced cancer referred to a phase I clinical trials program. J Clin Oncol. 2011;29:3547–3552. - PubMed
-
- Fu S, Barber FD, Naing A, et al. Advance care planning in patients with cancer referred to a phase I clinical trials program: The MD Anderson Cancer Center experience. J Clin Oncol. 2012;30:2891–2896. - PubMed
-
- Roberts TG, Jr., Goulart BH, Squitieri L, et al. Trends in the risks and benefits to patients with cancer participating in phase 1 clinical trials. JAMA. 2004;292:2130–2140. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

