The direction and role of phenotypic transition between podocytes and parietal epithelial cells in focal segmental glomerulosclerosis
- PMID: 24154691
- PMCID: PMC3921819
- DOI: 10.1152/ajprenal.00228.2013
The direction and role of phenotypic transition between podocytes and parietal epithelial cells in focal segmental glomerulosclerosis
Abstract
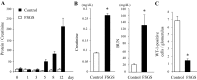
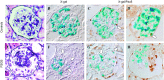
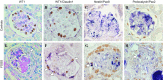
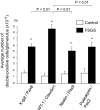
Focal segmental glomerulosclerosis (FSGS) is a podocyte disease. Among the various histologies of FSGS, active epithelial changes, hyperplasia, as typically seen in the collapsing variant, indicates disease progression. Using a podocyte-specific injury model of FSGS carrying a genetic podocyte tag combined with double immunostaining by different sets of podocytes and parietal epithelial cell (PEC) markers [nestin/Pax8, Wilms' tumor-1 (WT1)/claudin1, and podocalyxin/Pax2], we investigated the direction of epithelial phenotypic transition and its role in FSGS. FSGS mice showed progressive proteinuria and renal dysfunction often accompanied by epithelial hyperplasia, wherein 5-bromo-4-chloro-3-indoyl β-d-galactoside (X-gal)-positive podocyte-tagged cells were markedly decreased. The average numbers of double-positive cells in all sets of markers were significantly increased in the FSGS mice compared with the controls. In addition, the average numbers of double-positive cells for X-gal/Pax8, nestin/Pax8 and podocalyxin/Pax2 staining in the FSGS mice were comparable, whereas those of WT1/claudin1 were significantly increased. When we divided glomeruli from FSGS mice into those with FSGS lesions and those without, double-positive cells tended to be more closely associated with glomeruli without FSGS lesions compared with those with FSGS lesions. Moreover, the majority of double-positive cells appeared to be isolated and very rarely associated with FSGS lesions (1/1,997 glomeruli). This study is the first to show the incidence and localization of epithelial cells with phenotypical changes in FSGS using a genetic tag. The results suggest that the major direction of epithelial phenotypic transition in cellular FSGS is from podocytes to PECs and that these cells were less represented in the active lesions of FSGS.
Keywords: cellular FSGS; focal segmental glomerulosclerosis; parietal epithelial cell; phenotypic transition; podocyte.
Figures
Similar articles
-
Genetic podocyte lineage reveals progressive podocytopenia with parietal cell hyperplasia in a murine model of cellular/collapsing focal segmental glomerulosclerosis.Am J Pathol. 2009 May;174(5):1675-82. doi: 10.2353/ajpath.2009.080789. Epub 2009 Apr 9. Am J Pathol. 2009. PMID: 19359523 Free PMC article.
-
Aberrant Notch1-dependent effects on glomerular parietal epithelial cells promotes collapsing focal segmental glomerulosclerosis with progressive podocyte loss.Kidney Int. 2013 Jun;83(6):1065-75. doi: 10.1038/ki.2013.48. Epub 2013 Feb 27. Kidney Int. 2013. PMID: 23447065 Free PMC article.
-
Significance of early phenotypic change of glomerular podocytes detected by Pax2 in primary focal segmental glomerulosclerosis.Am J Kidney Dis. 2002 Mar;39(3):475-85. doi: 10.1053/ajkd.2002.31391. Am J Kidney Dis. 2002. PMID: 11877566
-
Mechanisms of Scarring in Focal Segmental Glomerulosclerosis.J Histochem Cytochem. 2019 Sep;67(9):623-632. doi: 10.1369/0022155419850170. Epub 2019 May 22. J Histochem Cytochem. 2019. PMID: 31116068 Free PMC article. Review.
-
Parietal Epithelial Cell Behavior and Its Modulation by microRNA-193a.Biomolecules. 2023 Jan 31;13(2):266. doi: 10.3390/biom13020266. Biomolecules. 2023. PMID: 36830635 Free PMC article. Review.
Cited by
-
Glomerular parietal epithelial cells contribute to adult podocyte regeneration in experimental focal segmental glomerulosclerosis.Kidney Int. 2015 Nov;88(5):999-1012. doi: 10.1038/ki.2015.152. Epub 2015 May 20. Kidney Int. 2015. PMID: 25993321 Free PMC article.
-
Transgenic Strategies to Study Podocyte Loss and Regeneration.Stem Cells Int. 2015;2015:678347. doi: 10.1155/2015/678347. Epub 2015 May 18. Stem Cells Int. 2015. PMID: 26089920 Free PMC article. Review.
-
The use of lineage tracing to study kidney injury and regeneration.Nat Rev Nephrol. 2015 Jul;11(7):420-31. doi: 10.1038/nrneph.2015.67. Epub 2015 May 12. Nat Rev Nephrol. 2015. PMID: 25963592 Review.
-
Morphological Features of Minimal Change Disease and Focal Segmental Glomerulosclerosis Using Repeat Biopsy and Parietal Epithelial Cell Marker.Kidney Dis (Basel). 2020 Mar;6(2):119-124. doi: 10.1159/000505125. Epub 2020 Jan 31. Kidney Dis (Basel). 2020. PMID: 32309294 Free PMC article.
-
APOL1 and kidney cell function.Am J Physiol Renal Physiol. 2019 Aug 1;317(2):F463-F477. doi: 10.1152/ajprenal.00233.2019. Epub 2019 Jun 26. Am J Physiol Renal Physiol. 2019. PMID: 31241995 Free PMC article. Review.
References
-
- Barisoni L, Kriz W, Mundel P, D'Agati V. The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 10: 51–61, 1999 - PubMed
-
- Bariéty J, Bruneval P, Hill G, Irinopoulou T, Mandet C, Meyrier A. Posttransplantation relapse of FSGS is characterized by glomerular epithelial cell transdifferentiation. J Am Soc Nephrol 2: 261–274, 2001 - PubMed
-
- D'Agati VD. Podocyte injury in focal segmental glomerulosclerosis: lessons from animal models. Kidney Int 73: 399–406, 2008 - PubMed
-
- D'Agati VD, Kaskel FJ, Falk RJ. Focal segmental glomerulosclerosis. N Engl J Med 365: 2398–2411, 2011 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

