A role for the circadian clock protein Per1 in the regulation of aldosterone levels and renal Na+ retention
- PMID: 24154698
- PMCID: PMC3882450
- DOI: 10.1152/ajprenal.00472.2013
A role for the circadian clock protein Per1 in the regulation of aldosterone levels and renal Na+ retention
Abstract
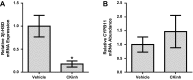
The circadian clock plays an important role in the regulation of physiological processes, including renal function and blood pressure. We have previously shown that the circadian protein period (Per)1 regulates the expression of multiple Na(+) transport genes in the collecting duct, including the α-subunit of the renal epithelial Na(+) channel. Consistent with this finding, Per1 knockout mice exhibit dramatically lower blood pressure than wild-type mice. We have also recently demonstrated the potential opposing actions of cryptochrome (Cry)2 on Per1 target genes. Recent work by others has demonstrated that Cry1/2 regulates aldosterone production through increased expression of the adrenal gland-specific rate-limiting enzyme 3β-dehydrogenase isomerase (3β-HSD). Therefore, we tested the hypothesis that Per1 plays a role in the regulation of aldosterone levels and renal Na(+) retention. Using RNA silencing and pharmacological blockade of Per1 nuclear entry in the NCI-H295R human adrenal cell line, we showed that Per1 regulates 3β-HSD expression in vitro. These results were confirmed in vivo: mice with reduced levels of Per1 had decreased levels of plasma aldosterone and decreased mRNA expression of 3β-HSD. We postulated that mice with reduced Per1 would have a renal Na(+)-retaining defect. Indeed, metabolic cage experiments demonstrated that Per1 heterozygotes excreted more urinary Na(+) compared with wild-type mice. Taken together, these data support the hypothesis that Per1 regulates aldosterone levels and that Per1 plays an integral role in the regulation of Na(+) retention.
Keywords: aldosterone; circadian clock; kidney; period 1; sodium transport.
Figures






Similar articles
-
Kidney-specific KO of the circadian clock protein PER1 alters renal Na+ handling, aldosterone levels, and kidney/adrenal gene expression.Am J Physiol Renal Physiol. 2022 Apr 1;322(4):F449-F459. doi: 10.1152/ajprenal.00385.2021. Epub 2022 Feb 7. Am J Physiol Renal Physiol. 2022. PMID: 35129370 Free PMC article.
-
Opposing actions of Per1 and Cry2 in the regulation of Per1 target gene expression in the liver and kidney.Am J Physiol Regul Integr Comp Physiol. 2013 Oct 1;305(7):R735-47. doi: 10.1152/ajpregu.00195.2013. Epub 2013 Jul 3. Am J Physiol Regul Integr Comp Physiol. 2013. PMID: 23824961 Free PMC article.
-
Transcriptional regulation of NHE3 and SGLT1 by the circadian clock protein Per1 in proximal tubule cells.Am J Physiol Renal Physiol. 2015 Dec 1;309(11):F933-42. doi: 10.1152/ajprenal.00197.2014. Epub 2015 Sep 16. Am J Physiol Renal Physiol. 2015. PMID: 26377793 Free PMC article.
-
Clock genes and salt-sensitive hypertension: a new type of aldosterone-synthesizing enzyme controlled by the circadian clock and angiotensin II.Hypertens Res. 2016 Oct;39(10):681-687. doi: 10.1038/hr.2016.91. Epub 2016 Jul 21. Hypertens Res. 2016. PMID: 27439492 Review.
-
Hypertension due to loss of clock: novel insight from the molecular analysis of Cry1/Cry2-deleted mice.Curr Hypertens Rep. 2011 Apr;13(2):103-8. doi: 10.1007/s11906-011-0181-3. Curr Hypertens Rep. 2011. PMID: 21286865 Review.
Cited by
-
Time-Restricted Eating in Metabolic Syndrome-Focus on Blood Pressure Outcomes.Curr Hypertens Rep. 2022 Nov;24(11):485-497. doi: 10.1007/s11906-022-01219-z. Epub 2022 Sep 6. Curr Hypertens Rep. 2022. PMID: 36066740 Free PMC article. Review.
-
Circadian regulation of renal function.Free Radic Biol Med. 2018 May 1;119:93-107. doi: 10.1016/j.freeradbiomed.2018.01.018. Epub 2018 Jan 31. Free Radic Biol Med. 2018. PMID: 29360554 Free PMC article. Review.
-
Current perspective on circadian function of the kidney.Am J Physiol Renal Physiol. 2024 Mar 1;326(3):F438-F459. doi: 10.1152/ajprenal.00247.2023. Epub 2023 Dec 22. Am J Physiol Renal Physiol. 2024. PMID: 38134232 Free PMC article. Review.
-
Desoxycorticosterone pivalate-salt treatment leads to non-dipping hypertension in Per1 knockout mice.Acta Physiol (Oxf). 2017 May;220(1):72-82. doi: 10.1111/apha.12804. Epub 2016 Oct 3. Acta Physiol (Oxf). 2017. PMID: 27636900 Free PMC article.
-
Chronobiology in nephrology: the influence of circadian rhythms on renal handling of drugs and renal disease treatment.Int Urol Nephrol. 2018 Dec;50(12):2221-2228. doi: 10.1007/s11255-018-2001-z. Epub 2018 Oct 15. Int Urol Nephrol. 2018. PMID: 30324579 Review.
References
-
- Albrecht U. The mammalian circadian clock: a network of gene expression. Front Biosci 9: 48–55, 2004 - PubMed
-
- Badura L, Swanson T, Adamowicz W, Adams J, Cianfrogna J, Fisher K, Holland J, Kleiman R, Nelson F, Reynolds L, St Germain K, Schaeffer E, Tate B, Sprouse J. An inhibitor of casein kinase I epsilon induces phase delays in circadian rhythms under free-running and entrained conditions. J Pharmacol Exp Ther 322: 730–738, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

