Prefusion structure of trimeric HIV-1 envelope glycoprotein determined by cryo-electron microscopy
- PMID: 24154805
- PMCID: PMC3917492
- DOI: 10.1038/nsmb.2711
Prefusion structure of trimeric HIV-1 envelope glycoprotein determined by cryo-electron microscopy
Abstract
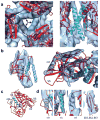
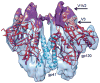
The activation of trimeric HIV-1 envelope glycoprotein (Env) by its binding to the cell-surface receptor CD4 and co-receptors (CCR5 or CXCR4) represents the first of a series of events that lead to fusion between viral and target-cell membranes. Here, we present the cryo-EM structure, at subnanometer resolution (~6 Å at 0.143 FSC), of the 'closed', prefusion state of trimeric HIV-1 Env complexed to the broadly neutralizing antibody VRC03. We show that three gp41 helices at the core of the trimer serve as an anchor around which the rest of Env is reorganized upon activation to the 'open' quaternary conformation. The architecture of trimeric HIV-1 Env in the prefusion state and in the activated intermediate state resembles the corresponding states of influenza hemagglutinin trimers, thus providing direct evidence for the similarity in entry mechanisms used by HIV-1, influenza and related enveloped viruses.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


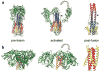
References
-
- Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280:1884–1888. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

