NADPH oxidase enzymes in skin fibrosis: molecular targets and therapeutic agents
- PMID: 24155025
- PMCID: PMC4043188
- DOI: 10.1007/s00403-013-1416-8
NADPH oxidase enzymes in skin fibrosis: molecular targets and therapeutic agents
Abstract
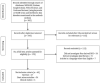
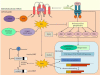
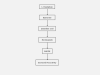
Fibrosis is characterized by the excessive deposition of extracellular matrix components eventually resulting in organ dysfunction and failure. In dermatology, fibrosis is the hallmark component of many skin diseases, including systemic sclerosis, graft-versus-host disease, hypertrophic scars, keloids, nephrogenic systemic fibrosis, porphyria cutanea tarda, restrictive dermopathy and other conditions. Fibrotic skin disorders may be debilitating and impair quality of life. There are few FDA-approved anti-fibrotic drugs; thus, research in this area is crucial in addressing this deficiency. Recent investigations elucidating the pathogenesis of skin fibrosis have implicated endogenous reactive oxygen species produced by the multicomponent nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Nox) enzyme complex. In this review, we discuss Nox enzymes and their role in skin fibrosis. An overview of the Nox enzyme family is presented and their role in the pathogenesis of skin fibrosis is discussed. The mechanisms by which Nox enzymes influence specific fibrotic skin disorders are also reviewed. Finally, we describe the therapeutic approaches to ameliorate skin fibrosis by directly targeting Nox enzymes with the use of statins, p47phox subunit modulators, or GKT137831, a competitive inhibitor of Nox enzymes. Nox enzymes can also be targeted indirectly via scavenging ROS with antioxidants. We believe that Nox modulators are worthy of further investigation and have the potential to transform the management of skin fibrosis by dermatologists.
Figures

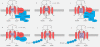
References
-
- Abdullah A, Blakeney P, Hunt R, Broemeling L, Phillips L, Herndon DN, Robson MC. Visible scars and self-esteem in pediatric patients with burns. The Journal of burn care & rehabilitation. 1994;15(2):164–168. - PubMed
-
- Akasaki T, Ohya Y, Kuroda J, Eto K, Abe I, Sumimoto H, Iida M. Increased expression of gp91phox homologues of NAD(P)H oxidase in the aortic media during chronic hypertension: involvement of the renin-angiotensin system. Hypertension research : official journal of the Japanese Society of Hypertension. 2006;29(10):813–820. doi:10.1291/hypres.29.813. - PubMed
-
- Alexandre J, Hu Y, Lu W, Pelicano H, Huang P. Novel action of paclitaxel against cancer cells: bystander effect mediated by reactive oxygen species. Cancer research. 2007;67(8):3512–3517. doi:10.1158/0008-5472.CAN-06-3914. - PubMed
-
- Altenhofer S, Kleikers PW, Radermacher KA, Scheurer P, Rob Hermans JJ, Schiffers P, Ho H, Wingler K, Schmidt HH. The NOX toolbox: validating the role of NADPH oxidases in physiology and disease. Cellular and molecular life sciences : CMLS. 2012;69(14):2327–2343. doi:10.1007/s00018-012-1010-9. - PMC - PubMed
-
- Aoyama T, Paik YH, Watanabe S, Laleu B, Gaggini F, Fioraso-Cartier L, Molango S, Heitz F, Merlot C, Szyndralewiez C, Page P, Brenner DA. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology. 2012;56(6):2316–2327. doi:10.1002/hep.25938. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

