Longitudinal analysis of HIV-1 coreceptor tropism by single and triplicate HIV-1 RNA and DNA sequencing in patients undergoing successful first-line antiretroviral therapy
- PMID: 24155059
- PMCID: PMC3954119
- DOI: 10.1093/jac/dkt426
Longitudinal analysis of HIV-1 coreceptor tropism by single and triplicate HIV-1 RNA and DNA sequencing in patients undergoing successful first-line antiretroviral therapy
Abstract
Objectives: Maraviroc has been shown to be effective in patients harbouring CCR5-tropic HIV-1. While this CCR5 antagonist has initially been used in salvage therapy, its excellent safety profile makes it ideal for antiretroviral treatment simplification strategies in patients with suppressed plasma viraemia. The aim of this study was to compare HIV-1 tropism as detected in baseline plasma RNA and peripheral blood mononuclear cell (PBMC) DNA prior to first-line therapy and to analyse tropism evolution while on successful treatment.
Methods: HIV-1 tropism was determined using triplicate genotypic testing combined with geno2pheno[coreceptor] analysis at a 10% false positive rate in 42 patients. Paired pre-treatment plasma RNA and PBMC DNA and two subsequent PBMC DNA samples (the first obtained after reaching undetectable plasma HIV-1 RNA and the second after at least 2 years of suppression of plasma viraemia) were evaluated.
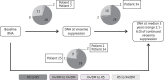
Results: Coreceptor tropism was completely concordant in paired pre-treatment RNA and DNA, with 26.2% of HIV-1 sequences predicted to be non-CCR5-tropic. During follow-up, coreceptor tropism switches were detected in 4 (9.5%) patients without any preferential direction. Although false positive rate discrepancies within triplicates were common, the rate of discordance of coreceptor tropism assignment among triplicate results in this mostly CCR5-tropic dataset was only 2.1%, questioning the added value of triplicate testing compared with single testing.
Conclusions: HIV-1 coreceptor tropism changes during virologically successful first-line treatment are infrequent. HIV-1 DNA analysis may thus support the choice of a CCR5 antagonist in treatment switch strategies; however, maraviroc treatment outcome data are required to confirm this option.
Keywords: HIV type 1; V3; genotype interpretation; gp120.
Figures

References
-
- Perry CM. Maraviroc: a review of its use in the management of CCR5-tropic HIV-1 infection. Drugs. 2010;70:1189–213. - PubMed
-
- Vandekerckhove LP, Wensing AM, Kaiser R, et al. European guidelines on the clinical management of HIV-1 tropism testing. Lancet Infect Dis. 2011;11:394–407. - PubMed
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services; 12 February 2013 http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. (16 October 2013, date last accessed)
-
- Soriano V, Perno CF, Kaiser R, et al. When and how to use maraviroc in HIV-infected patients. AIDS. 2009;23:2377–85. - PubMed
-
- Wasmuth JC, Rockstroh JK, Hardy WD. Drug safety evaluation of maraviroc for the treatment of HIV infection. Expert Opin Drug Saf. 2012;11:161–74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

