Functional consequences of complementarity-determining region deactivation in a multifunctional anti-nucleic acid antibody
- PMID: 24155236
- PMCID: PMC3861637
- DOI: 10.1074/jbc.M113.508499
Functional consequences of complementarity-determining region deactivation in a multifunctional anti-nucleic acid antibody
Abstract
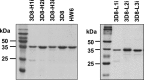
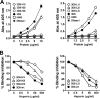
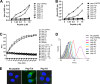
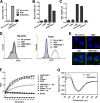
Many murine monoclonal anti-DNA antibodies (Abs) derived from mice models for systemic lupus erythematosus have additional cell-penetration and/or nucleic acid-hydrolysis properties. Here, we examined the influence of deactivating each complementarity-determining region (CDR) within a multifunctional anti-nucleic acid antibody (Ab) that possesses these activities, the catalytic 3D8 single chain variable fragment (scFv). CDR-deactivated 3D8 scFv variants were generated by replacing all of the amino acids within each CDR with Gly/Ser residues. The structure of 3D8 scFv accommodated single complete CDR deactivations. Different functional activities of 3D8 scFv were affected differently depending on which CDR was deactivated. The only exception was CDR1, located within the light chain (LCDR1); deactivation of LCDR1 abolished all of the functional activities of 3D8 scFv. A hybrid Ab, HW6/3D8L1, in which the LCDR1 from an unrelated Ab (HW6) was replaced with the LCDR1 from 3D8, acquired all activities associated with the 3D8 scFv. These results suggest that the activity of a multifunctional 3D8 scFv Ab can be modulated by single complete CDR deactivation and that the LCDR1 plays a crucial role in maintaining Ab properties. This study presents a new approach for determining the role of individual CDRs in multifunctional Abs with important implications for the future of Ab engineering.
Keywords: Anti-DNA Antibody; Antibodies; Antibody Engineering; Autoimmunity; CDR Deactivation; Cell Penetration; DNA Hydrolysis; Immunology; Molecular Biology; Multifunctional Antibody.
Figures



Similar articles
-
A Tat-grafted anti-nucleic acid antibody acquires nuclear-localization property and a preference for TAR RNA.Biochem Biophys Res Commun. 2011 Mar 18;406(3):403-7. doi: 10.1016/j.bbrc.2011.02.054. Epub 2011 Feb 15. Biochem Biophys Res Commun. 2011. PMID: 21329654
-
Heavy and light chain variable single domains of an anti-DNA binding antibody hydrolyze both double- and single-stranded DNAs without sequence specificity.J Biol Chem. 2006 Jun 2;281(22):15287-95. doi: 10.1074/jbc.M600937200. Epub 2006 Mar 20. J Biol Chem. 2006. PMID: 16551636
-
Generation of a chickenized catalytic anti-nucleic acid antibody by complementarity-determining region grafting.Mol Immunol. 2015 Feb;63(2):513-20. doi: 10.1016/j.molimm.2014.10.009. Mol Immunol. 2015. PMID: 25458312
-
Understanding the Significance and Implications of Antibody Numbering and Antigen-Binding Surface/Residue Definition.Front Immunol. 2018 Oct 16;9:2278. doi: 10.3389/fimmu.2018.02278. eCollection 2018. Front Immunol. 2018. PMID: 30386328 Free PMC article. Review.
-
SDR grafting--a new approach to antibody humanization.Methods. 2005 May;36(1):25-34. doi: 10.1016/j.ymeth.2005.01.003. Methods. 2005. PMID: 15848072 Review.
Cited by
-
In-Cell RNA Hydrolysis Assay: A Method for the Determination of the RNase Activity of Potential RNases.Mol Biotechnol. 2015 Jun;57(6):506-12. doi: 10.1007/s12033-015-9844-7. Mol Biotechnol. 2015. PMID: 25632893 Free PMC article.
-
Computational de novo design of antibodies binding to a peptide with high affinity.Biotechnol Bioeng. 2017 Jun;114(6):1331-1342. doi: 10.1002/bit.26244. Epub 2017 Feb 2. Biotechnol Bioeng. 2017. PMID: 28059445 Free PMC article.
-
Heparan sulfate proteoglycans (HSPGs) and chondroitin sulfate proteoglycans (CSPGs) function as endocytic receptors for an internalizing anti-nucleic acid antibody.Sci Rep. 2017 Oct 30;7(1):14373. doi: 10.1038/s41598-017-14793-z. Sci Rep. 2017. PMID: 29085061 Free PMC article.
-
DNA-histone complexes as ligands amplify cell penetration and nuclear targeting of anti-DNA antibodies via energy-independent mechanisms.Immunology. 2016 Jan;147(1):73-81. doi: 10.1111/imm.12542. Epub 2015 Nov 24. Immunology. 2016. PMID: 26447818 Free PMC article.
-
Cytoplasmic delivery of antibodies through grafting a functional single complementarity-determining region loop.FEBS Lett. 2025 May;599(10):1442-1455. doi: 10.1002/1873-3468.70058. Epub 2025 May 1. FEBS Lett. 2025. PMID: 40313010 Free PMC article.
References
-
- Putterman C. (2004) New approaches to the renal pathogenicity of anti-DNA antibodies in systemic lupus erythematosus. Autoimmun. Rev. 3, 7–11 - PubMed
-
- Schroeder K., Herrmann M., Winkler T. H. (2013) The role of somatic hypermutation in the generation of pathogenic antibodies in SLE. Autoimmunity 46, 121–127 - PubMed
-
- Rahman A., Latchman D. S., Isenberg D. A. (1998) Immunoglobulin variable region sequences of human monoclonal anti-DNA antibodies. Semin. Arthritis Rheum. 28, 141–154 - PubMed
-
- Radic M. Z., Weigert M. (1994) Genetic and structural evidence for antigen selection of anti-DNA antibodies. Annu. Rev. Immunol. 12, 487–520 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

