Soluble guanylate cyclase generation of cGMP regulates migration of MGE neurons
- PMID: 24155296
- PMCID: PMC3866415
- DOI: 10.1523/JNEUROSCI.1871-13.2013
Soluble guanylate cyclase generation of cGMP regulates migration of MGE neurons
Abstract
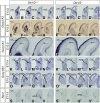
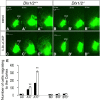
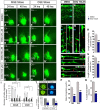
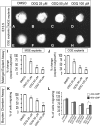
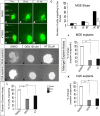
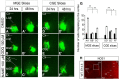
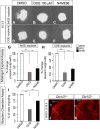
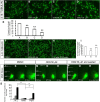
Here we have provided evidence that nitric oxide-cyclic GMP (NO-cGMP) signaling regulates neurite length and migration of immature neurons derived from the medial ganglionic eminence (MGE). Dlx1/2(-/-) and Lhx6(-/-) mouse mutants, which exhibit MGE interneuron migration defects, have reduced expression of the gene encoding the α subunit of a soluble guanylate cyclase (Gucy1A3). Furthermore, Dlx1/2(-/-) mouse mutants have reduced expression of NO synthase 1 (NOS1). Gucy1A3(-/-) mice have a transient reduction in cortical interneuron number. Pharmacological inhibition of soluble guanylate cyclase and NOS activity rapidly induces neurite retraction of MGE cells in vitro and in slice culture and robustly inhibits cell migration from the MGE and caudal ganglionic eminence. We provide evidence that these cellular phenotypes are mediated by activation of the Rho signaling pathway and inhibition of myosin light chain phosphatase activity.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
