Regulator of calcineurin 1 modulates expression of innate anxiety and anxiogenic responses to selective serotonin reuptake inhibitor treatment
- PMID: 24155299
- PMCID: PMC3807023
- DOI: 10.1523/JNEUROSCI.3513-12.2013
Regulator of calcineurin 1 modulates expression of innate anxiety and anxiogenic responses to selective serotonin reuptake inhibitor treatment
Abstract
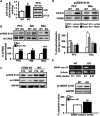
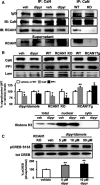
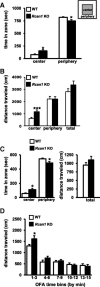
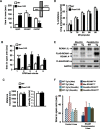
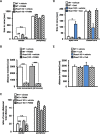
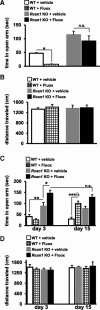
Regulator of calcineurin 1 (RCAN1) controls the activity of calcium/calmodulin-dependent phosphatase calcineurin (CaN), which has been implicated in human anxiety disorders. Previously, we reported that RCAN1 functioned as an inhibitor of CaN activity in the brain. However, we now find enhanced phosphorylation of a CaN substrate, cAMP response element-binding protein (CREB), in the brains of Rcan1 knock-out (KO) mice. Consistent with enhanced CREB activation, we also observe enhanced expression of a CREB transcriptional target, brain-derived neurotrophic factor (BDNF) in Rcan1 KO mice. We also discovered that RCAN1 deletion or blockade of RCAN1-CaN interaction reduced CaN and protein phosphatase-1 localization to nuclear-enriched protein fractions and promoted CREB activation. Because of the potential links between CREB, BDNF, and anxiety, we examined the role of RCAN1 in the expression of innate anxiety. Rcan1 KO mice displayed reduced anxiety in several tests of unconditioned anxiety. Acute pharmacological inhibition of CaN rescued these deficits while transgenic overexpression of human RCAN1 increased anxiety. Finally, we found that Rcan1 KO mice lacked the early anxiogenic response to the selective serotonin reuptake inhibitor (SSRI) fluoxetine and had improved latency for its therapeutic anxiolytic effects. Together, our study suggests that RCAN1 plays an important role in the expression of anxiety-related and SSRI-related behaviors through CaN-dependent signaling pathways. These results identify RCAN1 as a mediator of innate emotional states and possible therapeutic target for anxiety.
Figures
Similar articles
-
The regulator of calcineurin 1 (RCAN1/DSCR1) activates the cAMP response element-binding protein (CREB) pathway.J Biol Chem. 2011 Oct 28;286(43):37841-8. doi: 10.1074/jbc.M111.232165. Epub 2011 Sep 2. J Biol Chem. 2011. PMID: 21890628 Free PMC article.
-
CREB activates proteasomal degradation of DSCR1/RCAN1.FEBS Lett. 2008 Jun 11;582(13):1889-93. doi: 10.1016/j.febslet.2008.04.059. Epub 2008 May 15. FEBS Lett. 2008. PMID: 18485898
-
Regulation of RCAN1 protein activity by Dyrk1A protein-mediated phosphorylation.J Biol Chem. 2011 Nov 18;286(46):40401-12. doi: 10.1074/jbc.M111.253971. Epub 2011 Sep 30. J Biol Chem. 2011. PMID: 21965663 Free PMC article.
-
RCAN1-mediated calcineurin inhibition as a target for cancer therapy.Mol Med. 2022 Jun 18;28(1):69. doi: 10.1186/s10020-022-00492-7. Mol Med. 2022. PMID: 35717152 Free PMC article. Review.
-
Chronic high levels of the RCAN1-1 protein may promote neurodegeneration and Alzheimer disease.Free Radic Biol Med. 2013 Sep;62:47-51. doi: 10.1016/j.freeradbiomed.2013.01.016. Epub 2013 Jan 29. Free Radic Biol Med. 2013. PMID: 23369757 Free PMC article. Review.
Cited by
-
RCAN1 knockout and overexpression recapitulate an ensemble of rest-activity and circadian disruptions characteristic of Down syndrome, Alzheimer's disease, and normative aging.J Neurodev Disord. 2022 May 24;14(1):33. doi: 10.1186/s11689-022-09444-y. J Neurodev Disord. 2022. PMID: 35610565 Free PMC article.
-
Exercise alters mouse sperm small noncoding RNAs and induces a transgenerational modification of male offspring conditioned fear and anxiety.Transl Psychiatry. 2017 May 2;7(5):e1114. doi: 10.1038/tp.2017.82. Transl Psychiatry. 2017. PMID: 28463242 Free PMC article.
-
The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring.Science. 2016 Feb 26;351(6276):933-9. doi: 10.1126/science.aad0314. Epub 2016 Jan 28. Science. 2016. PMID: 26822608 Free PMC article.
-
Combination effects of a fatty diet and exercise on the depressive state and cardioprotection in apolipoprotein E knockout mice with a change in RCAN1 expression.J Int Med Res. 2020 Nov;48(11):300060520964016. doi: 10.1177/0300060520964016. J Int Med Res. 2020. PMID: 33251902 Free PMC article.
-
Down syndrome and Alzheimer's disease: common molecular traits beyond the amyloid precursor protein.Aging (Albany NY). 2020 Jan 9;12(1):1011-1033. doi: 10.18632/aging.102677. Epub 2020 Jan 9. Aging (Albany NY). 2020. PMID: 31918411 Free PMC article. Review.
References
-
- Baldwin DS, Tiwari N. The pharmacologic treatment of patients with generalized anxiety disorder: where are we now and where are we going? CNS Spectr. 2009;14:5–12. - PubMed
-
- Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, Impey S, Storm DR, Neve RL, Yin JC, Zachariou V, Nestler EJ. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci U S A. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
