Deletion of CPEB3 enhances hippocampus-dependent memory via increasing expressions of PSD95 and NMDA receptors
- PMID: 24155305
- PMCID: PMC6618447
- DOI: 10.1523/JNEUROSCI.3043-13.2013
Deletion of CPEB3 enhances hippocampus-dependent memory via increasing expressions of PSD95 and NMDA receptors
Abstract
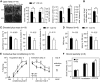
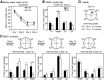
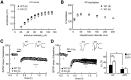
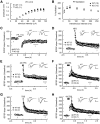
Long-term memory requires activity-dependent synthesis of plasticity-related proteins (PRPs) to strengthen synaptic efficacy and consequently consolidate memory. Cytoplasmic polyadenylation element binding protein (CPEB)3 is a sequence-specific RNA-binding protein that regulates translation of several PRP RNAs in neurons. To understand whether CPEB3 plays a part in learning and memory, we generated CPEB3 knock-out (KO) mice and found that the null mice exhibited enhanced hippocampus-dependent, short-term fear memory in the contextual fear conditioning test and long-term spatial memory in the Morris water maze. The basal synaptic transmission of Schaffer collateral-CA1 neurons was normal but long-term depression evoked by paired-pulse low-frequency stimulation was modestly facilitated in the juvenile KO mice. Molecular and cellular characterizations revealed several molecules in regulating plasticity of glutamatergic synapses are translationally elevated in the CPEB3 KO neurons, including the scaffolding protein PSD95 and the NMDA receptors along with the known CPEB3 target, GluA1. Together, CPEB3 functions as a negative regulator to confine the strength of glutamatergic synapses by downregulating the expression of multiple PRPs and plays a role underlying certain forms of hippocampus-dependent memories.
Figures





Similar articles
-
Elevated activation of CaMKIIα in the CPEB3-knockout hippocampus impairs a specific form of NMDAR-dependent synaptic depotentiation.Front Cell Neurosci. 2014 Nov 3;8:367. doi: 10.3389/fncel.2014.00367. eCollection 2014. Front Cell Neurosci. 2014. PMID: 25404896 Free PMC article.
-
Deletion of glutamate delta-1 receptor in mouse leads to enhanced working memory and deficit in fear conditioning.PLoS One. 2013;8(4):e60785. doi: 10.1371/journal.pone.0060785. Epub 2013 Apr 3. PLoS One. 2013. PMID: 23560106 Free PMC article.
-
Impaired synaptic clustering of postsynaptic density proteins and altered signal transmission in hippocampal neurons, and disrupted learning behavior in PDZ1 and PDZ2 ligand binding-deficient PSD-95 knockin mice.Mol Brain. 2012 Dec 26;5:43. doi: 10.1186/1756-6606-5-43. Mol Brain. 2012. PMID: 23268962 Free PMC article.
-
A Place at the Table: LTD as a Mediator of Memory Genesis.Neuroscientist. 2016 Aug;22(4):359-71. doi: 10.1177/1073858415588498. Epub 2015 May 20. Neuroscientist. 2016. PMID: 25993993 Review.
-
Role of CPEB3 protein in learning and memory: new insights from synaptic plasticity.Aging (Albany NY). 2020 Jul 2;12(14):15169-15182. doi: 10.18632/aging.103404. Epub 2020 Jul 2. Aging (Albany NY). 2020. PMID: 32619199 Free PMC article. Review.
Cited by
-
Prenatal diesel exhaust exposure alters hippocampal synaptic plasticity in offspring.Aging (Albany NY). 2024 Mar 1;16(5):4348-4362. doi: 10.18632/aging.205592. Epub 2024 Mar 1. Aging (Albany NY). 2024. PMID: 38431308 Free PMC article.
-
Learning induces the translin/trax RNase complex to express activin receptors for persistent memory.Elife. 2017 Sep 20;6:e27872. doi: 10.7554/eLife.27872. Elife. 2017. PMID: 28927503 Free PMC article.
-
CPEB3 can regulate seizure susceptibility by inhibiting the transcriptional activity of STAT3 on NMDARs expression.Mol Med. 2025 Feb 24;31(1):77. doi: 10.1186/s10020-025-01136-2. Mol Med. 2025. PMID: 39994587 Free PMC article.
-
Comparative analyses of vertebrate CPEB proteins define two subfamilies with coordinated yet distinct functions in post-transcriptional gene regulation.Genome Biol. 2022 Sep 12;23(1):192. doi: 10.1186/s13059-022-02759-y. Genome Biol. 2022. PMID: 36096799 Free PMC article.
-
Adiponectin receptor-mediated signaling ameliorates cerebral cell damage and regulates the neurogenesis of neural stem cells at high glucose concentrations: an in vivo and in vitro study.Cell Death Dis. 2015 Aug 6;6(8):e1844. doi: 10.1038/cddis.2015.220. Cell Death Dis. 2015. PMID: 26247729 Free PMC article.
References
-
- Brigman JL, Wright T, Talani G, Prasad-Mulcare S, Jinde S, Seabold GK, Mathur P, Davis MI, Bock R, Gustin RM, Colbran RJ, Alvarez VA, Nakazawa K, Delpire E, Lovinger DM, Holmes A. Loss of GluN2B-containing NMDA receptors in CA1 hippocampus and cortex impairs long-term depression, reduces dendritic spine density, and disrupts learning. J Neurosci. 2010;30:4590–4600. doi: 10.1523/JNEUROSCI.0640-10.2010. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
